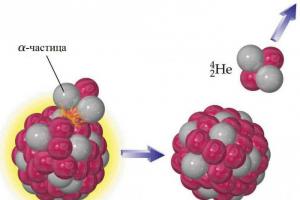
Containing two protons and two neutrons.
Encyclopedic YouTube
1 / 5
✪ Helium is a SUPERFLUID AND COLDEST ELEMENT!
✪ Superfluid helium. University of Stuttgart
✪ Prospects for thermonuclear energy (physicist Anton Tyulusov tells)
✪ Operation "Helium"
✪ Operation "Helium". Episode 3
Subtitles
I want to recommend you Andrey's channel on he is making a video course on organic chemistry for grade 10, now more than 40 videos on 12 topics are available on his channel, subscribe to Andrey's channel to publish and play for 100 points and so today I will tell you about the most common noble gas in the observable universe, which can also acquire unique superfluid properties at extremely low temperatures, meet helium in the periodic table, this element is located in the upper right corner, it is very easy to find at number 2, I think that people today become familiar with this inert gas from childhood due to its lightness relative to air, helium is excellent for inflating holiday balloons, which children love so much, this is all due to the fact that the molar mass of helium is approximately seven times less than the molar mass of air, but still, in terms of prevalence, gels on earth are extremely rare in the air, they are only found only one part per million, the bulk of the resulting helium for the same balls comes from natural gas, in which the concentration of helium can reach up to seven percent by weight, all because as a result of the radioactive decay of uranium or thorium in the earth's crust, helium can accumulate in underground voids with natural gas and not evaporate into the atmosphere, however, if we take it on a larger scale, then in the entire observable universe, or it will take an honorable second place in abundance among all elements, second only to hydrogen and forming about a quarter of all atoms, just imagine that all the atoms heavier the gel forms only two percent of the mass of the total mass of matter, here you can feel how small we are on the scale of the universe; the main part is actually found in stars or in the atmosphere of gas giants, which, like the entire universe, contain about 20 percent of the mass, according to today's data, the main part of the gel is located in space was formed during the big bang about 14 billion years ago, let's now return from heaven to earth and consider the properties of this gas in a more tangible experiment I have a small ampoule of helium which is at a very low pressure of about one hundredth of atmospheric pressure, it is clear that the gel is not has colors besides this, it still has no taste or smell, you could find this out if you have ever tried to breathe this gas, however, such experiments are extremely dangerous since our cells do not breathe helium, they need oxygen for this, this even forced the current sellers of gel balloons adding up to 20 percent oxygen to them that you hung at parties has become safer if you pass a high-frequency high-voltage discharge through the oculus with gel, it will begin to glow in a dull orange color, the brightness of which will depend on the voltage and on the diameter of the ampoule I used a DPL generator as a voltage source I knew about and what gave me the opportunity to hold the ampoule directly in my hand and due to the presence of electrical capacity in my body, in principle, like any other, unlike it on or xenon, helium lights up already at a distance from the generator wire since it has less ionization energy, unfortunately with chemical point of view, it doesn’t really have any interesting properties; it doesn’t react with practically any substance, although still in the form of plasma it looks like what you see in an ampoule. Gels can form an extremely unstable compound with hydrogen, deuterium, or some metals, and at high pressures of thousands of atmospheres. even special substances are formed, clart from and helios nitrogen, which in the form of crystals can be grown on diamond substrates, it’s a pity that all these substances are very unstable and they are almost impossible to see under normal conditions, but there is no need to be upset because the gel has the most interesting and unique physical properties of all gases. is that when cooled to a temperature of 42 Kelvin, it actually becomes the lightest and coldest liquid whose density is almost 10 times less than the density of water in degrees Celsius; liquid helium is obtained at crazy minus two hundred and sixty-eight degrees, which is very cold, so cold that some metals at such temperature becomes superconductors, for example, mercury or niobium, in order to maintain such a low temperature, liquid helium is located in a double Dewar vessel, which is also cooled from the outside with liquid nitrogen. The same technology for cooling liquid helium is used in modern devices to create nuclear magnetic resonance; in them superconductors, the niobium compound is cooled with liquid helium which, due to its high cost, is in turn cooled with cheaper liquid nitrogen, thus a liquid gel and serves medicine as well as for scientific research, but the most interesting is yet to come. Before that, I told you about the first form of liquid helium, the so-called helium 1, if you start cooling it by lowering the pressure in the vessel, the liquid helium will eventually pass into the so-called
Prevalence
Opening
The existence of helium-3 was proposed by Australian scientist Mark Oliphant while working at the University of Cambridge in . This isotope was finally discovered by Luis Alvarez and Robert Cornog.
Physical properties
Receipt
Currently, helium-3 is not obtained from natural sources (minuscule amounts of helium-3 are available on Earth, extremely difficult to obtain), but is created from the decay of artificially produced tritium.
Price
The average price of helium-3 in 2009 was, according to some estimates, about 930 USD per liter.
Plans for mining helium-3 on the Moon
Helium-3 is a byproduct of reactions occurring on the Sun and is found in some quantities in the solar wind and the interplanetary medium. Helium-3 entering the Earth's atmosphere from interplanetary space quickly dissipates back, its concentration in the atmosphere is extremely low
Hypothetically, during thermonuclear fusion, when 1 ton of helium-3 reacts with 0.67 tons of deuterium, energy is released equivalent to the combustion of 15 million tons of oil (however, the technical feasibility of this reaction has not been studied at the moment). Consequently, the lunar resource of helium-3 (according to maximum estimates) could last about five millennia for the population of our planet. The main problem remains the reality of extracting helium from lunar regolith. As mentioned above, the content of helium-3 in regolith is ~1 g per 100 tons. Therefore, to extract a ton of this isotope, at least 100 million tons of soil must be processed in situ.
Usage
Neutron counters
Gas meters filled with helium-3 are used to detect neutrons. This is the most common method for measuring neutron flux. There is a reaction in them
n+ 3 He → 3 H + 1 H + 0.764 MeV.The charged reaction products - triton and proton - are recorded by a gas counter operating in the mode of a proportional counter or Geiger-Muller counter.
Receiving ultra-low temperatures
By dissolving liquid helium-3 in helium-4, millikelvin temperatures are reached.
Medicine
Helium-3 as nuclear fuel
The reaction 3 He + D → 4 He + p has a number of advantages compared to the deuterium-tritium reaction T + D → 4 He + n, which is most achievable under terrestrial conditions. These benefits include:
- Tens of times lower neutron flux from the reaction zone, which sharply reduces induced radioactivity and degradation of reactor structural materials;
- The resulting protons, unlike neutrons, are easily captured and can be used for additional generation of electricity, for example, in an MHD generator;
- The starting materials for the synthesis are inactive and their storage does not require special precautions;
- In the event of a reactor accident with depressurization of the core, the radioactivity of the release is close to zero.
The disadvantages of the helium-deuterium reaction include a significantly higher temperature threshold. It is necessary to reach a temperature of approximately 10 9 K due to the Coulomb barrier for it to start. And at a lower temperature, the thermonuclear reaction of the fusion of deuterium nuclei with each other proceeds much more readily, and the reaction between deuterium and helium-3 does not occur.
In art
In science fiction works (games, films, anime), helium-3 sometimes acts as the main fuel and as a valuable resource, including mined on the Moon.
The plot of the 2009 British science fiction film Moon 2112 is based on the operation of the Lunar mining complex. The complex ensures the production of the helium-3 isotope, with the help of which it was possible to stop the catastrophic energy crisis on Earth.
In the political comedy Iron Sky, lunar helium-3 became the cause of an international nuclear conflict over mining rights.
In the anime " Planetes» helium-3 is used as fuel for rocket engines, etc.
Literature
- Dobbs E. R. Helium Three. - Oxford University press, 2000. ISBN 0-19-850640-6
- Galimov E. M. If you have energy, you can extract everything - Rare earths. 2014. No. 2. P. 6-12.
- The Helium-3 Shortage: Supply, Demand, and Options for Congress // FAS, December 22, 2010 (English)
Notes
- Audi G., Wapstra A. H., Thibault C.
Helium is an inert gas of the 18th group of the periodic table. It is the second lightest element after hydrogen. Helium is a colorless, odorless and tasteless gas that becomes liquid at a temperature of -268.9 °C. Its boiling and freezing points are lower than those of any other known substance. It is the only element that does not harden when cooled at normal atmospheric pressure. For helium to turn into a solid state, 25 atmospheres are required at a temperature of 1 K.
History of discovery
Helium was discovered in the gaseous atmosphere surrounding the Sun by French astronomer Pierre Jansen, who in 1868, during an eclipse, discovered a bright yellow line in the spectrum of the solar chromosphere. This line was originally thought to represent the element sodium. In the same year, English astronomer Joseph Norman Lockyer observed a yellow line in the solar spectrum that did not correspond to the known D 1 and D 2 lines of sodium, and therefore he called it the D 3 line. Lockyer concluded that it was caused by a substance in the Sun that was unknown on Earth. He and chemist Edward Frankland used the Greek name for the Sun, helios, to name the element.
In 1895, British chemist Sir William Ramsay proved the existence of helium on Earth. He obtained a sample of the uranium-bearing mineral kleveite, and after examining the gases produced by heating it, he discovered that the bright yellow line in the spectrum coincided with the D 3 line observed in the spectrum of the Sun. Thus, the new element was finally installed. In 1903, Ramsay and Frederic Soddu determined that helium was a product of the spontaneous decay of radioactive substances.
Distribution in nature
The mass of helium makes up about 23% of the total mass of the universe, and the element is the second most abundant in space. It is concentrated in stars, where it is formed from hydrogen as a result of thermonuclear fusion. Although helium is found in the earth's atmosphere at a concentration of 1 part in 200 thousand (5 ppm) and is found in small quantities in radioactive minerals, meteorite iron, and mineral springs, large quantities of the element are found in the United States (especially in Texas, New Mexico, Kansas, Oklahoma, Arizona and Utah) as a component (up to 7.6%) of natural gas. Small reserves have been discovered in Australia, Algeria, Poland, Qatar and Russia. In the earth's crust, the concentration of helium is only about 8 parts per billion.
Isotopes
The nucleus of each helium atom contains two protons, but like other elements, it has isotopes. They contain from one to six neutrons, so their mass numbers range from three to eight. The stable ones are the elements in which the mass of helium is determined by the atomic numbers 3 (3 He) and 4 (4 He). All the rest are radioactive and very quickly decay into other substances. Terrestrial helium is not an original component of the planet; it was formed as a result of radioactive decay. Alpha particles emitted by nuclei of heavy radioactive substances are nuclei of the isotope 4 He. Helium does not accumulate in large quantities in the atmosphere because Earth's gravity is not strong enough to prevent it from gradually leaking into space. Traces of 3 He on Earth are explained by the negative beta decay of the rare element hydrogen-3 (tritium). 4 He is the most abundant of the stable isotopes: the ratio of 4 He to 3 He atoms is about 700 thousand to 1 in the atmosphere and about 7 million to 1 in some helium-containing minerals.

Physical properties of helium
This element has the lowest boiling and melting points. For this reason, helium exists except in extreme conditions. He gas dissolves less in water than any other gas, and the rate of diffusion through solids is three times greater than that of air. Its refractive index is closest to 1.
The thermal conductivity of helium is second only to that of hydrogen, and its specific heat capacity is unusually high. At normal temperatures it heats up as it expands, and below 40 K it cools down. Therefore, at T<40 K гелий можно превратить в жидкость путем расширения.
An element is a dielectric unless it is in an ionized state. Like other noble gases, helium has metastable energy levels that allow it to remain ionized in an electrical discharge when the voltage remains below the ionization potential.
Helium-4 is unique in that it has two liquid forms. The common one is called helium I and exists at temperatures ranging from a boiling point of 4.21 K (-268.9 °C) to about 2.18 K (-271 °C). Below 2.18 K, the thermal conductivity of 4 He becomes 1000 times greater than that of copper. This form is called helium II to distinguish it from the normal form. It is superfluid: the viscosity is so low that it cannot be measured. Helium II spreads into a thin film on the surface of any substance it touches, and this film flows without friction, even against gravity.
The less abundant helium-3 forms three different liquid phases, two of which are superfluids. Superfluidity in 4 He was discovered by a Soviet physicist in the mid-1930s, and the same phenomenon in 3 He was first noticed by Douglas D. Osheroff, David M. Lee, and Robert S. Richardson of the United States in 1972.
A liquid mixture of two isotopes of helium-3 and -4 at temperatures below 0.8 K (-272.4 °C) is divided into two layers - almost pure 3 He and a mixture of 4 He with 6% helium-3. The dissolution of 3 He into 4 He is accompanied by a cooling effect, which is used in the design of cryostats in which the temperature of helium drops below 0.01 K (-273.14 °C) and is maintained there for several days.

Connections
Under normal conditions, helium is chemically inert. In extreme cases, it is possible to create element compounds that are not stable at normal temperatures and pressures. For example, helium can form compounds with iodine, tungsten, fluorine, phosphorus and sulfur when it is exposed to an electrical glow discharge by bombardment with electrons or in a plasma state. Thus, HeNe, HgHe 10, WHe 2 and the molecular ions He 2 +, He 2 ++, HeH + and HeD + were created. This technique also made it possible to obtain neutral He 2 and HgHe molecules.
Plasma
Ionized helium is predominantly distributed in the Universe, the properties of which differ significantly from molecular helium. Its electrons and protons are not bound, and it has very high electrical conductivity even in a partially ionized state. Charged particles are strongly affected by magnetic and electric fields. For example, in the solar wind, helium ions along with ionized hydrogen interact with the Earth's magnetosphere, causing the northern lights.

Discovery of deposits in the USA
After drilling a well in 1903 in Dexter, Kansas, non-flammable gas was obtained. Initially it was not known that it contained helium. What kind of gas was found was determined by state geologist Erasmus Haworth, who collected samples of it and at the University of Kansas, with the help of chemists Cady Hamilton and David McFarland, found that it contained 72% nitrogen, 15% methane, 1% hydrogen and 12% was not identified. After further analysis, the scientists found that 1.84% of the sample was helium. This is how they learned that this chemical element is present in huge quantities in the depths of the Great Plains, from where it can be extracted from natural gas.
Industrial production
This made the United States the leader in world helium production. At the suggestion of Sir Richard Threlfall, the US Navy funded three small experimental plants to produce this substance during the First World War, with the aim of providing barrage balloons with a lightweight, non-flammable lifting gas. This program produced a total of 5,700 m 3 of 92 percent He, although only less than 100 liters of gas had previously been produced. Some of this volume was used in the world's first helium airship, the C-7, which made its maiden voyage from Hampton Roads to Bolling Field on December 7, 1921.
Although the process of low-temperature gas liquefaction was not sufficiently developed at the time to prove significant during World War I, production continued. Helium was primarily used as a lifting gas in aircraft. Demand for it increased during World War II when it was used in shielded arc welding. The element was also important in the Manhattan atomic bomb project.

US National Stockpile
In 1925, the United States government established the National Helium Reserve in Amarillo, Texas, to supply military airships in times of war and commercial airships in times of peace. Use of the gas declined after World War II, but the supply was increased in the 1950s to supply, among other things, a coolant used in the production of oxyhydrogen rocket fuel during the space race and the Cold War. US helium use in 1965 was eight times the peak wartime consumption.
After the passage of the Helium Act of 1960, the Bureau of Mines contracted 5 private enterprises to extract the element from natural gas. For this program, a 425-kilometer natural gas pipeline was built to connect these plants to a government-owned partially depleted gas field near Amarillo, Texas. The helium-nitrogen mixture was pumped into an underground storage facility and remained there until it was needed.
By 1995, a billion cubic meter reserve had been collected and the National Reserve was $1.4 billion in debt, prompting the US Congress to phase it out in 1996. Following the passage of the helium privatization law in 1996, the Ministry of Natural Resources began dismantling the storage facility in 2005.

Purity and production volumes
Helium produced before 1945 was about 98% pure, the remaining 2% being nitrogen, which was sufficient for airships. In 1945, a small amount of 99.9 percent gas was produced for use in arc welding. By 1949, the purity of the resulting element reached 99.995%.
For many years, the United States produced more than 90% of the world's commercial helium. Since 2004, 140 million m 3 have been produced annually, 85% of which comes from the USA, 10% was produced in Algeria, and the rest in Russia and Poland. The main sources of helium in the world are gas fields in Texas, Oklahoma and Kansas.
Receipt process
Helium (98.2% pure) is separated from natural gas by liquefying other components at low temperatures and high pressures. Adsorption of other gases by cooled activated carbon allows a purity of 99.995% to be achieved. A small amount of helium is produced by liquefying air on a large scale. From 900 tons of air you can get about 3.17 cubic meters. m of gas.

Areas of application
Noble gas has found application in various fields.
- Helium, whose properties make it possible to obtain ultra-low temperatures, is used as a cooling agent in the Large Hadron Collider, superconducting magnets in MRI machines and nuclear magnetic resonance spectrometers, satellite equipment, as well as for liquefying oxygen and hydrogen in Apollo rockets.
- As an inert gas for welding aluminum and other metals, in the production of optical fibers and semiconductors.
- To create pressure in the fuel tanks of rocket engines, especially those that run on liquid hydrogen, since only gaseous helium retains its state of aggregation when hydrogen remains liquid);
- He-Ne is used to scan barcodes at supermarket checkout counters.
- A helium ion microscope produces better images than an electron microscope.
- Due to its high permeability, noble gas is used to check for leaks, for example in car air conditioning systems, and to quickly inflate airbags in the event of a collision.
- Low density allows you to fill decorative balloons with helium. Inert gas replaced explosive hydrogen in airships and balloons. For example, in meteorology, helium balloons are used to lift measuring instruments.
- In cryogenic technology it serves as a coolant, since the temperature of this chemical element in the liquid state is the lowest possible.
- Helium, whose properties provide it with low reactivity and solubility in water (and blood), mixed with oxygen, has found use in breathing compositions for scuba diving and caisson work.
- Meteorites and rocks are analyzed for the content of this element to determine their age.
Helium: properties of the element
The main physical properties of He are as follows:
- Atomic number: 2.
- Relative mass of helium atom: 4.0026.
- Melting point: no.
- Boiling point: -268.9 °C.
- Density (1 atm, 0 °C): 0.1785 g/p.
- Oxidation states: 0.
“We are now talking about thermonuclear energy of the future and a new ecological type of fuel that cannot be produced on Earth. We are talking about the industrial development of the Moon for the extraction of helium-3.” This statement by the head of the Energia rocket and space corporation, Nikolai Sevastyanov, if it did not shake the imagination of law-abiding Russians (now, just on the eve of the new heating season, they only have to deal with helium-3), then the imagination of specialists and interested people did not leave them indifferent.
This is understandable: given, to put it mildly, the not brilliant state of affairs in the domestic aerospace industry (Russia’s space budget is 30 times less than in the USA and 2 times less than in India; from 1989 to 2004 we launched only 3 research spacecraft), suddenly, like this, no more, no less - the Russians will mine helium-3 on the Moon! Let me remind you that, theoretically, this light isotope of helium is capable of entering into a thermonuclear reaction with deuterium. Accordingly, many scientists consider fusion to be a potentially limitless source of cheap energy. However, there is a problem: helium-3 makes up less than one millionth of the total amount of helium on Earth. But in the lunar soil this light isotope is found in abundance: according to academician Eric Galimov, about 500 million tons...
They say that at one time in the USA there was a huge poster in front of the entrance to Disneyland: “We and our country can do anything, the only thing that limits us is the boundaries of our imagination.” All this was not far from the truth: a fast and effective atomic project, a fantastically successful lunar program, a strategic defense initiative (SDI), which completely destroyed the Soviet economy. ...
Essentially, one of the main functions of the state, especially in the 20th century, was precisely to formulate tasks beyond the imagination for the scientific community. This also applies to the Soviet state: electrification, industrialization, the creation of the atomic bomb, the first satellite, the turning of rivers... By the way, we also had our own “poster” in front of Disneyland - “We were born to make a fairy tale come true!”
“I just think that there is a shortage in some major technological problem,” Alexander Zakharov, Doctor of Physical and Mathematical Sciences, Scientific Secretary of the Space Research Institute of the Russian Academy of Sciences, emphasized in a conversation with me. “Maybe this is why all this talk about mining helium-3 on the Moon for thermonuclear energy has arisen recently. If the Moon is a source of minerals, and this helium-3 is brought from there, and there is not enough energy on Earth... All this is understandable, it sounds very beautiful. And it may be easy to persuade influential people to allocate money for this. I think so".
But the whole point is that now on Earth there is no technology - and in the next at least 50 years its appearance is not expected - burning helium-3 in a thermonuclear reaction. There is not even a preliminary design for such a reactor. The international thermonuclear reactor ITER, currently under construction in France, is designed to “burn” hydrogen isotopes – deuterium and tritium. The estimated temperature for “ignition” of a thermonuclear reaction is 100–200 million degrees. To use helium-3, the temperature must be an order of magnitude or two higher.
So, the head of Russia's largest rocket and space corporation, Nikolai Sevastyanov, excuse the expression, is fooling us with his helium-3? Does not look like it. For what!?
“The space industry is naturally interested in such a large and expensive project,” says Alexander Zakharov. “But from the point of view of its practical use, it is absolutely obvious that this is premature.”
To implement the helium-3 project, it is necessary to create a special program for additional research of the Moon, launch an entire squadron of spacecraft, resolve issues with the extraction of helium-3, its processing... This will ruin the country worse than any SDI.
“I don’t want to say that the Moon is completely closed from a scientific point of view - there are still scientific tasks there,” emphasizes Alexander Zakharov. – But, as they say, this needs to be done step by step, I don’t forget about other scientific tasks. Otherwise, we somehow shy away: as soon as the Americans announced a program for a manned flight to Mars, we immediately declared that we were also ready to do this. We heard about the lunar programs - let’s do this too... We don’t have a deliberate, balanced, strategic national task.”
Here we are again back to where we started - to the strategic national task. The trouble is that, unlike Americans, we are limited not so much by our imagination - with this, as Nikolai Sevastyanov’s statement shows, we are all right. But the “helium-3” program (let’s call it that), according to the most conservative estimates, will require $5 billion for five years of research.
From a purely scientific point of view, there has been some stagnation in the problem of fusion based on TOKAMAKs, even despite the decision made to build the international experimental reactor ITER. (However, this is a topic for a separate discussion.) It seems to me that the helium-3 problem for some of the influential thermonuclear lobby is a new niche for resuscitation and the realization of professional ambitions.
Moreover - and this is a completely sensational thing, and that’s the only reason I didn’t start my article with it - as an expert from the aerospace industry told us, $1 billion has been allocated for the Russian project for the extraction of a light helium isotope on the Moon! This money is supposedly of American origin.
Despite the intricacy of such a combination, the ends meet quite successfully. In order to achieve the allocation of $104 billion for the recently announced program to create a lunar base, the US National Aeronautics and Space Administration had to show that “strategic competitors” are also not asleep. That is, the “Russian” billion is, in a way, overhead costs for NASA... Hence the surge of interest in the production of helium-3 in Russia, inexplicable by rational motives.
If this is really the case, then once again we will all have to be convinced of the validity of the formula published ten years ago in the journal Physics Today. Here it is: “Scientists are not disinterested seekers of truth, but rather participants in an intense competition for scientific influence, the winners of which break the bank.”
It will not take long, by the standards of human civilization, before fossil natural resources will be exhausted. Among the possible candidates for replacing oil and gas are solar energy, wind power, or hydrogen. In recent years, you can increasingly hear about a new salvation for the planet called helium-3. It was only recently discovered that this substance can be used as a raw material for power plants.
General information about the substance: properties
In 1934, Australian physicist Mark Oliphant, while working at the Cavendish Laboratory at the University of Cambridge in England, came to a remarkable discovery. During the first demonstration of nuclear fusion by bombarding a deuteron target, he hypothesized the existence of a new isotope of the chemical element number 2. Today it is known as helium-3.
It has the following properties:
- Contains two protons, one neutron and two electrons;
- Among all known elements, it is the only stable isotope that has more protons than neutrons;
- Boils at 3.19 Kelvin (-269.96 degrees Celsius). During boiling, a substance loses half its density;
- The angular momentum is ½, making it a fermion;
- The latent heat of vaporization is 0.026 KJ/mol;
Five years after the discovery of Mark Oliphant, his theoretical constructions received experimental confirmation. And after 9 years, scientists managed to obtain a compound V liquid form . As it turned out, in this state of aggregation, helium-3 has superfluid properties.
In other words, at temperatures close to absolute zero, it is able to penetrate through capillaries and narrow cracks, experiencing virtually no resistance from friction.

Helium-3 mining on the Moon
Over billions of years, the solar wind deposited gigantic amounts of helium-3 into the surface layer of regolith. According to estimates, its amount on the earth's satellite can reach 10 million tons.
Many space powers have a program for extracting this substance for the purpose of subsequent thermonuclear fusion:
- In January 2006, the Russian company Energia announced plans to begin geological work on the Moon by 2020. Today, the future of the project is in limbo due to the difficult economic situation of the country;
- In 2008, the Indian Space Research Organization sent a probe to the surface of the earth's satellite, one of the goals of which was stated to be the study of helium-containing minerals;
- China also has its own plans for deposits of precious raw materials. According to plans, it is planned to send three shuttles to the satellite annually. The energy produced from this fuel will more than cover the needs of all humanity.
For now it remains a dream that can only be seen in science fiction films. Among them are “Moon” (2009) and “Iron Sky” (2012).
In this video, physicist Boris Romanov will tell you in what form the substance helium-3 is found on the Moon, and whether it is possible to import it from there:
Geochemical data
The isotope is also present on planet Earth, although in smaller quantities:
- This is the main component of the earth's mantle, which was synthesized during planet formation. Its total mass in this part of the planet is, according to various estimates, from 0.1 to 1 million tons;
- It comes to the surface as a result of volcanic activity. Thus, the hills of the Hawaiian Islands emit about 300 grams of this substance per year. Mid-ocean ridges - about 3 kilograms;
- In places where one lithospheric plate collides with another, there may be hundreds of thousands of tons of helium isotope. It is not possible to extract this wealth industrially at the present stage of technological development;
- Nature continues to produce this compound to this day, as a result of the decay of radioactive elements in the crust and mantle;
- It can be found in fairly small quantities (up to 0.5%) in some natural gas sources. As experts note, every year during the transportation of natural gas, 26 m 3 of helium-3 is separated;
- It is also present in the earth's atmosphere. Its specific fraction is approximately 7.2 parts per trillion atoms of other atmospheric gases. According to the latest calculations, the total mass of atmospheric 3 2 he reaches at least 37 thousand tons.

Modern uses of the substance
Almost all of the isotopes used in the national economy are produced by the radioactive decay of tritium, which is bombarded with lithium-6 neutrons in a nuclear reactor.
For decades helium-3 was just a by-product in the manufacture of atomic weapon warheads. However, after the signing of the START I treaty in 1991, the superpowers reduced the volume of missile production, which is why production products also began to decline.
Today, production of the isotope is booming as new uses have been found for it:
- Due to the relatively high gyromagnetic ratio, particles of this substance are used in medical tomography of the lungs. The patient inhales a gas mixture containing hyperpolarized helium-3 atoms. Then, under the influence of infrared laser radiation, the computer draws anatomical and functional images of the organs;
- In scientific laboratories, this compound is used for cryogenic purposes. By evaporating it from the surface of the refrigerator, it is possible to achieve values close to 0.2 kelvin;
- In recent years, the idea of using the substance as a feedstock for power plants has been gaining popularity. The first such installation was built in 2010 in the Tennessee Valley (USA).

Helium-3 as a fuel
A second, revised approach to the use of controlled fusion energy involves the use of 3 2 he and deuterium as raw materials. The result of such a reaction will be helium-4 ion and high-energy protons.
Theoretically, this technology has the following advantages:
- High efficiency because an electrostatic field is used to control the fusion of ions. The kinetic energy of protons is directly converted into electricity through solid-state conversion. There is no need to build turbines, which are used in nuclear power plants to convert the energy of protons into heat;
- Lower, in comparison with other types of power plants, capital and operating costs;
- Neither air nor water is polluted;
- Relatively small dimensions due to the use of modern compact installations;
- There is no radioactive fuel.
However, critics note the significant “crudeness” of this decision. At best commercial use of thermonuclear fusion will begin no earlier than 2050.
Among all the isotopes of a chemical element with atomic number 2, helium-3 stands out. What it is can be briefly described by the following properties: it is stable (that is, it does not undergo transformations as a result of radiation), has superfluid properties in liquid form, and has a relatively small mass.

Video about the formation of helium-3 in the Universe
In this video, physicist Daniil Potapov will tell you how helium-3 was formed in the Universe, what role it played in the formation of the Universe:
Helium-three. A strange and incomprehensible phrase. Nevertheless, the further we go, the more we will hear it. Because, according to experts, it is helium-three that will save our world from the impending energy crisis. And in this enterprise the most active role is assigned to Russia.
Moon

Promising thermonuclear energy, using the deuterium-tritium fusion reaction as a basis, although safer than the energy of nuclear fission, which is used in modern nuclear power plants, still has a number of significant drawbacks.
- Firstly, this reaction releases a much larger (by an order of magnitude!) number of high-energy neutrons. None of the known materials can withstand such an intense neutron flux for more than six years - despite the fact that it makes sense to make a reactor with a resource of at least 30 years. Consequently, the first wall of a tritium fusion reactor will need to be replaced - and this is a very complex and expensive procedure, which also involves shutting down the reactor for a fairly long period of time.
- Secondly, it is necessary to shield the magnetic system of the reactor from powerful neutron radiation, which complicates and, accordingly, increases the cost of the design.
- Third, many structural elements of a tritium reactor after the end of operation will be highly active and will require long-term burial in storage facilities specially created for this purpose.
In the case of using deuterium with the helium-3 isotope instead of tritium in a thermonuclear reactor, most problems can be solved. The intensity of the neutron flux drops by 30 times - accordingly, a service life of 30-40 years can be easily ensured. After the end of operation of the helium reactor, no high-level waste will be generated, and the radioactivity of the structural elements will be so low that they can be literally buried in a city landfill, lightly sprinkled with earth.

What's the problem? Why are we still not using such beneficial thermonuclear fuel?
First of all, because this isotope is extremely scarce on our planet. It is born in the Sun, which is why it is sometimes called a “solar isotope.” Its total mass there exceeds the weight of our planet. Helium-3 is carried into the surrounding space by the solar wind. The Earth's magnetic field deflects a significant part of this wind, and therefore helium-3 makes up only one trillionth of the Earth's atmosphere - about 4000 tons. On the Earth itself it is even less - about 500 kg.
There is much more of this isotope on the Moon. There it is embedded in the lunar soil “regolith”, whose composition resembles ordinary slag. We are talking about huge - almost inexhaustible reserves!
Analysis of six soil samples brought by the Apollo expeditions and two samples delivered by Soviet automatic stations " Moon“, showed that the regolith covering all the seas and plateaus of the Moon contains up to 106 tons of helium-3, which would meet the needs of earthly energy, even increased several times compared to modern ones, for a millennium! According to modern estimates, the reserves of helium-3 on the Moon are three orders of magnitude greater - 109 tons.
In addition to the Moon, helium-3 can be found in the dense atmospheres of the giant planets, and, according to theoretical estimates, its reserves on Jupiter alone amount to 1020 tons, which would be enough to power the Earth’s energy supply until the end of time.
Helium-3 mining projects
Regolith covers the Moon with a layer several meters thick. The regolith of the lunar seas is richer in helium than the regolith of the plateaus. 1 kg of helium-3 is contained in approximately 100,000 tons of regolith.
Therefore, in order to extract the precious isotope, it is necessary to process a huge amount of crumbly lunar soil.
Taking into account all the features, the helium-3 production technology should include the following processes:
1. Extraction of regolith.
Special “harvesters” will collect regolith from a surface layer about 2 m thick and deliver it to processing points or process it directly during the mining process.
2. Release of helium from regolith.
When the regolith is heated to 600?C, 75% of the helium contained in the regolith is released (desorbed); when heated to 800?C, almost all of the helium is released. It is proposed to heat the dust in special furnaces, focusing sunlight either with plastic lenses or mirrors.
3. Delivery to Earth by reusable spacecraft.
When helium-3 is mined, numerous substances are also extracted from the regolith: hydrogen, water, nitrogen, carbon dioxide, nitrogen, methane, carbon monoxide, which can be useful for maintaining the lunar industrial complex.
The project of the first lunar harvester, designed to process regolith and extract the helium-3 isotope from it, was proposed by the group of J. Kulczynski. Currently, private American companies are developing several prototypes, which, apparently, will be submitted to the competition after NASA decides on the features of a future expedition to the Moon.
It is clear that, in addition to delivering harvesters to the Moon, storage facilities, a manned base (to service the entire complex of equipment), a cosmodrome and much more will have to be built there. It is believed, however, that the high costs of creating a developed infrastructure on the Moon will pay off handsomely in terms of the coming global energy crisis, when traditional types of energy resources (coal, oil, natural gas) will have to be abandoned.
Main technological problem
There is one important problem on the way to creating energy based on helium-3. The fact is that the deuterium-helium-3 reaction is much more difficult to carry out than the deuterium-tritium reaction.
First of all, it is unusually difficult to ignite a mixture of these isotopes. The estimated temperature at which a thermonuclear reaction will occur in a deuterium-tritium mixture is 100-200 million degrees. When using helium-3, the required temperature is two orders of magnitude higher. In fact, we must light a small sun on Earth.
However, the history of the development of nuclear energy (the last half century) demonstrates an increase in generated temperatures by an order of magnitude within 10 years. In 1990, the European tokamak JET already burned helium-3, and the resulting power was 140 kW. Around the same time, the American tokamak TFTR reached the temperature necessary to start the reaction in the deuterium-helium mixture.
However, lighting the mixture is still half the battle. The downside of thermonuclear energy is the difficulty of obtaining practical returns, because the working fluid is plasma heated to many millions of degrees, which has to be kept in a magnetic field.
Experiments on taming plasma have been carried out for many decades, but only at the end of June last year in Moscow, representatives of a number of countries signed an agreement on the construction in the south of France in the city of Cadarache of the International Experimental Thermonuclear Reactor (ITER) - a prototype of a practical thermonuclear power plant. ITER will use deuterium and tritium as fuel.
A helium-3 thermonuclear reactor will be structurally more complex than ITER, and so far it is not even in the projects. And although experts hope that a prototype helium-3 reactor will appear in the next 20-30 years, for now this technology remains pure fantasy.
The issue of helium-3 mining was analyzed by experts during a hearing on the future of lunar exploration and development, held in April 2004 in the Subcommittee on Space and Aeronautics of the US House of Representatives Science Committee. Their conclusion was clear: even in the distant future, mining helium-3 on the Moon is completely unprofitable.
As John Logsdon, director of the Space Policy Institute in Washington, noted: “The US space community does not view helium-3 mining as a serious excuse for returning to the Moon. Flying there for this isotope is the same as sending Columbus to India for uranium five hundred years ago. He could bring it, and he would bring it, but for another few hundred years no one would know what to do with it.”
Helium-3 extraction as a national project

“We are now talking about thermonuclear energy of the future and a new ecological type of fuel that cannot be produced on Earth. We are talking about the industrial development of the Moon for the extraction of helium-3.”
This statement by the head of the Energia rocket and space corporation, Nikolai Sevastyanov, was perceived by Russian scientific observers as an application for the formation of a new “national project.”
Indeed, in fact, one of the main functions of the state, especially in the 20th century, was precisely the formulation of tasks for society on the verge of imagination. This also applied to the Soviet state: electrification, industrialization, the creation of the atomic bomb, the first satellite, the turning of rivers.
Today in the Russian Federation the state is trying, but cannot formulate tasks that are on the verge of the impossible. The state needs someone to show it a national project and justify the benefits that theoretically flow from this project. The program for the development and extraction of helium-3 from the Moon to Earth in order to supply thermonuclear energy with fuel ideally meets these requirements.
“I just think that there is a deficiency in some major technological problem,” Alexander Zakharov, Doctor of Physical and Mathematical Sciences, Scientific Secretary of the Space Research Institute of the Russian Academy of Sciences, emphasized in an interview. “Maybe this is why all this talk about mining helium-3 on the Moon for thermonuclear energy has arisen recently. If Moon- a source of minerals, and from there to bring this helium-3, but on Earth there is not enough energy... All this is understandable, it sounds very beautiful. And it may be easy to persuade influential people to allocate money for this. I think so".