If you find an error on a page, select it and press Ctrl + Enter
Preparation of phosphine
When white phosphorus is heated with a strong alkali solution, the phosphorus disproportionates, resulting in the formation of phosphate and phosphine PH 3. Simultaneously with phosphine, a small amount of diphosphine P 2 H 4 (a phosphorous analogue of hydrazine) is formed, which easily flares up in air. At the same time, hydrogen is formed. If the gas outlet tube is directed under water, phosphine bubbles flare up to the surface; This produces rings of white smoke.
Here is a description of the experience from the workshop Ripan R. Ceteanu I. Guide to practical work in inorganic chemistry .
Preparation of hydrogen phosphide by heating white phosphorus with a 30-50% solution of potassium hydroxide. Reaction equation:
4P + 3KOH + 3H 2 O = PH 3 + 3KH 2 PO 2
With this method of production, in addition to gaseous hydrogen phosphide, liquid hydrogen phosphide, gaseous hydrogen and acid potassium hypophosphite are also formed according to the equations:
6P + 4KOH + 4H 2 O = P 2 H 4 + 4KH 2 PO 2
2P + 2KOH + 2H 2 O = H 2 + 2KH 2 PO 2
Liquid hydrogen phosphide, interacting with potassium hydroxide in an aqueous environment, forms gaseous hydrogen phosphide, hydrogen and acid potassium hypophosphite according to the equations:
2P 2 H 4 + KOH + H 2 O = 3PH 3 + KH 2 PO 2
P 2 H 4 + 2KOH + 2H 2 O = 3H 2 + 2KH 2 PO 2
Acid potassium hypophosphite in an alkaline environment is converted into potassium orthophosphate with the release of hydrogen:
KH 2 PO 2 + 2KOH = 2H 2 + K 3 PO 4
According to the above reaction equations, when white phosphorus is heated with potassium hydroxide, gaseous hydrogen phosphide, hydrogen and potassium orthophosphate are formed.
The phosphine obtained in this way spontaneously ignites. This is because it contains some vapors of self-igniting liquid hydrogen phosphide (diphosphine) and hydrogen.
Instead of potassium hydroxide, you can use sodium, calcium or barium oxide hydrates. Reactions with them proceed in a similar way.
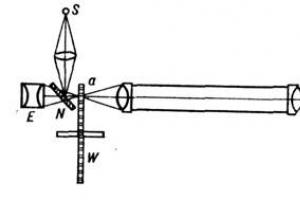
The device is a round-bottomed flask with a capacity of 100-250 ml, tightly closed with a rubber stopper, through which a tube must be tightly passed, directing gaseous products into a crystallizer with water.
The flask is filled to 3/4 of its volume with a 30-50% solution of caustic potassium, into which 2-3 pieces of white phosphorus, the size of a pea, are thrown. The flask is secured in a tripod clamp and, using a gas outlet tube, is connected to a crystallizer filled with water (see figure).
When the flask is heated, potassium hydroxide reacts with white phosphorus according to the above equations.
Liquid hydrogen phosphorous (diphosphine), upon reaching the surface of the liquid in the flask, immediately ignites and burns in the form of sparks; this occurs until the remaining oxygen in the flask is consumed.
When the flask is heated strongly, liquid hydrogen phosphide is distilled and gaseous hydrogen phosphide and hydrogen are ignited above the water. Phosphorous hydrogen burns with a yellow flame, producing phosphorus anhydride in the form of white smoke rings.
At the end of the experiment, reduce the flame under the flask, remove the plug with the outlet tube, stop heating and leave the device under the draft until it cools completely.
Unused phosphorus is thoroughly washed with water and stored for subsequent experiments.
We decided to get phosphine. Caustic soda was poured into a test tube and half filled with water. Some of the alkali remained in the sediment. The test tube was fixed obliquely in a stand, a pea-sized piece of yellow phosphorus was placed in it and closed with a stopper with a gas outlet tube, the end of which was lowered into a crystallizer with water. Started heating.
Gas bubbles began to bubble in the crystallizer. Over time, yellow flashes began, accompanied by popping sounds: the bubbles burst and caught fire in the air. After the outbreaks, beautiful white smoke rings often formed and rose upward.
According to our observations, the experiment worked best when the liquid in the test tube was actively boiling and some of the liquid was transferred into the water of the crystallizer. In some cases, it turned out that flashes occurred less frequently and weaker if the end of the gas outlet tube was lowered too deep into the water.
In general, the “fireworks with smoke rings” lasted up to several minutes. It's safe to say that this is one of the most beautiful experiences.
________________________________________
DEFINITION
Phosphine(phosphorus hydride, monophosphane) under normal conditions is a colorless gas, poorly soluble in water and does not react with it.
The gross formula is PH 3 (the structure of the molecule is shown in Fig. 1). The molar mass of phosphine is 34.00 g/mol.
Rice. 1. The structure of the phosphine molecule, indicating the bond angle and the length of the chemical bond.
At low temperatures it forms solid clarate 8PH 3 × 46H 2 O. Density - 1.5294 g/l. Boiling point - (-87.42 o C), melting point - (-133.8 o C).
In ORR it is a strong reducing agent, oxidized by concentrated sulfuric and nitric acids, iodine, oxygen, hydrogen peroxide, sodium hypochlorite. The donor properties are much less pronounced than those of ammonia.
PH3, oxidation states of elements in it
To determine the oxidation states of the elements that make up phosphine, you first need to understand for which elements this value is precisely known.
Phosphine is the trivial name for phosphorus hydride, and, as is known, the oxidation state of hydrogen in hydrides is (+1). To find the oxidation state of phosphorus, we take its value as “x” and determine it using the electrical neutrality equation:
x + 3×(+1) = 0;
This means the oxidation state of phosphorus in phosphine is (-3):
Examples of problem solving
EXAMPLE 1
| Exercise | Determine the oxidation states of acid-forming elements in the following compounds: HNO 2, H 2 CO 3, H 4 SiO 4, HPO 3. |
| Solution | In these compounds, the acid-forming elements are nitrogen, carbon, silicon and phosphorus. The oxidation state of oxygen is (-2), and that of hydrogen is (+1). Let’s take the oxidation state of the acid-forming element as “x” and use the electroneutrality equation to find its value: 1 + x + 2×(-2) = 0; The oxidation state of nitrogen is (+3). 2×(+1) + x + 3×(-2) = 0; The oxidation state of carbon is (+4). 4×(+1) + x + 4×(-2) = 0; The oxidation state of silicon is (+4). 1 + x + 3×(-2) = 0; The oxidation state of phosphorus is (+5). |
| Answer | HN +3 O 2 , H 2 C +4 O 3 , H 4 Si +4 O 4 , HP +5 O 3 |
EXAMPLE 2
| Exercise | Iron exhibits the highest degree of oxidation in the compound:
|
| Solution | In order to give the correct answer to the question posed, we will alternately determine the oxidation state of iron in each of the proposed compounds using the electroneutrality equation. a) The oxidation state of potassium is always equal to (+1). The oxidation state of carbon in the cyanide ion is (+2), and that of nitrogen is (-3). Let us take the value of the oxidation state of iron as “x”: 4×1 + x + 6×2 + 6× (-3) = 0; b) The oxidation state of potassium is always equal to (+1). The oxidation state of carbon in the cyanide ion is (+2), and that of nitrogen is (-3). Let us take the value of the oxidation state of iron as “x”: 3×1 + x + 6×2 + 6× (-3) = 0; c) The oxidation state of oxygen in oxides (-2). Let us take the value of the oxidation state of iron as “x”: d) The oxidation states of oxygen and hydrogen are (-2) and (+1), respectively. Let us take the value of the oxidation state of iron as “x”: x + 2×(-2) + 2× 1 = 0; The highest oxidation state of iron is (+3) and it manifests it in a compound with the composition K3. |
| Answer | Option 2 |
Chemistry tutor
Continuation. See in No. 22/2005; 1, 2, 3, 5, 6, 8, 9, 11, 13, 15, 16, 18, 22/2006;
3, 4, 7, 10, 11, 21/2007;
2, 7, 11, 18, 19, 21/2008;
1, 3, 10, 11/2009
LESSON 30
10th grade(first year of study)
Phosphorus and its compounds
1. Position in the table of D.I. Mendeleev, structure of the atom.
2. Brief history of the discovery and origin of the name.
3. Physical properties.
4. Chemical properties.
5. Being in nature.
6. Basic methods of obtaining
7. The most important phosphorus compounds.
Phosphorus is in the main subgroup of group V of D.I. Mendeleev’s periodic system. Its electronic formula is 1 s 2 2s 2 p 6 3s 2 p 3, this R-element. The characteristic oxidation states of phosphorus in compounds are –3, +3, +5; The most stable oxidation state is +5. In compounds, phosphorus can be part of both cations and anions, for example:

Phosphorus gets its name from the ability of white phosphorus to glow in the dark. The Greek word is translated as “bringer of light.” Phosphorus owes this name to its discoverer, the alchemist Brand, who, mesmerized by the glow of white phosphorus, came to the conclusion that he had received the philosopher's stone.
Phosphorus can exist in the form of several allotropic modifications, the most stable of which are white, red and black phosphorus.
Molecule white phosphorus (the most active allotrope) has a molecular crystal lattice, at the nodes of which there are tetraatomic P 4 molecules of a tetrahedral structure.
White phosphorus is soft, like wax, melts and boils without decomposition, and has a garlicky odor. In air, white phosphorus quickly oxidizes (glows greenish), and self-ignition of fine white phosphorus is possible. Insoluble in water (stored under a layer of water), but soluble in organic solvents. Toxic (even in small doses, MPC = 0.03 mg/m3). Has very high chemical activity. When heated without air access to 250–300 ° C, it turns into red phosphorus.
Red phosphorus – it is an inorganic polymer; macromolecules P n can have both a cyclic and acyclic structure. Its properties are sharply different from white phosphorus: it is not toxic, does not glow in the dark, does not dissolve in carbon disulfide and other organic solvents, and does not have high chemical activity. At room temperature it slowly turns into white phosphorus; when heated to 200 °C under pressure it turns into black phosphorus.
Black phosphorus looks like graphite. In structure, it is an inorganic polymer, the molecules of which have a layered structure. Semiconductor. Not poisonous. Chemical activity is significantly lower than that of white phosphorus. Stable in air. When heated, it turns into red phosphorus.
Chemical properties
The most chemically active is white phosphorus (but in practice they prefer to work with red phosphorus). It can exhibit the properties of both an oxidizing agent and a reducing agent in reactions, for example:
4P + 3O 2 2P 2 O 3,
4P + 5O 2 2P 2 O 5.
Metals (+/–)*:
3Ca + 2P Ca 3 P 2,
3Na + P Na 3 P,
Cu + P reaction does not occur.
Non-metals (+):
![]()
2P + 3I 2PI 3,
6P + 5N 2 2P 2 N 5 .

Basic oxides (–).
Acidic oxides (–).
Alkalis (+):
Acids (not oxidizing agents) (–).
Oxidizing acids (+):
3P (cr.) + 5HNO 3 (dil.) + 2H 2 O = 3H 3 PO 4 + 5NO,
P (red) + 5HNO 3 (conc.) H 3 PO 4 + 5NO 2 + H 2 O,
2P (cr.) + H 2 SO 4 (conc.) 2H 3 PO 4 + 5SO 2 + 2H 2 O.
Salts (–)**.
In nature, phosphorus occurs in the form of compounds (salts), the most important of which are phosphorite (Ca 3 (PO 4) 2), chlorapatite (Ca 3 (PO 4) 2 CaCl 2) and fluorapatite (Ca 3 ( PO 4) 2 CaF 2). Calcium phosphate is found in the bones of all vertebrates, causing their strength.
Phosphorus is obtained in electric furnaces by fusing calcium phosphate, sand and coal without access to air:
Ca 3 (PO 4) 2 + 3SiO 2 + 5C 2P + 5CO + 3CaSiO 3.
The most important phosphorus compounds include: phosphine, phosphorus(III) oxide, phosphorus(V) oxide, phosphoric acids.
F o f i n
This hydrogen compound of phosphorus, a colorless gas with a garlicky fishy odor, is highly poisonous. Poorly soluble in water, but highly soluble in organic solvents. Much less stable than ammonia, but is a stronger reducing agent. Has no practical significance.
To obtain phosphine, a direct synthesis reaction from simple substances is usually not used; The most common method for producing phosphine is the hydrolysis of phosphides:
Ca 3 P 2 + 6HOH = 3Ca(OH) 2 + 2PH 3.
In addition, phosphine can be obtained by a disproportionation reaction between phosphorus and alkali solutions:
4P + 3KOH + 3H 2 O PH 3 + KPO 2 H 2 ,
or from phosphonium salts:
PH 4 I PH 3 + HI,
PH 4 I + NaOH PH 3 + NaI + H 2 O.
It is advisable to consider the chemical properties of phosphine from two sides.
Acid-base properties. Phosphine forms an unstable hydrate with water, exhibiting very weak basic properties:
PH 3 + H 2 O PH 3 H 2 O (PH 4 OH),
PH 3 + HCl PH 4 Cl,
2PH 3 + H 2 SO 4 (PH 4) 2 SO 4.
Redox properties. Phosphine is a strong reducing agent:
2PH 3 + 4O 2 P 2 O 5 + 3H 2 O,
PH 3 + 8AgNO 3 + 4H 2 O = H 3 PO 4 + 8Ag + 8HNO 3.
O s i d p h o s p h o r a (III)
Oxide P 2 O 3 (true formula - P 4 O 6) is a white crystalline substance, a typical acidic oxide. When reacting with water in the cold, it forms phosphorous acid (medium strength):
P 2 O 3 + 3H 2 O = 2H 3 PO 3

Since phosphorous acid is dibasic, when phosphorus trioxide reacts with alkalis, two types of salts are formed - hydrophosphites and dihydrophosphites.
For example:
P 2 O 3 + 4NaOH = 2Na 2 HPO 3 + H 2 O,
P 2 O 3 + 2NaOH + H 2 O = 2NaH 2 PO 3.
Phosphorus dioxide P 2 O 3 is oxidized by atmospheric oxygen to pentoxide:
P 2 O 3 + O 2 P 2 O 5 .
Phosphorus trioxide and phosphorous acid are fairly strong reducing agents. Phosphorus(III) oxide is obtained by the slow oxidation of phosphorus in the absence of oxygen:
4P + 3O 2 2P 2 O 3 .
Phosphorus(V) oxides and phosphoric acids
Phosphorus pentoxide P 2 O 5 (true formula – P 4 O 10) is a white hygroscopic crystalline substance. In the solid and gaseous states, the molecule exists in the form of a dimer; at high temperatures it monomerizes. Typical acid oxide. It dissolves very well in water, forming a number of phosphoric acids:
metaphosphoric:
P 2 O 5 + H 2 O = 2HPO 3

pyrophosphoric (diphosphoric):
P 2 O 5 + 2H 2 O = H 4 P 2 O 7

orthophosphoric (phosphoric):
P 2 O 5 + 3H 2 O = 2H 3 PO 4

Phosphorus pentoxide exhibits all the properties characteristic of acid oxides, for example:
P 2 O 5 + 3H 2 O = 2H 3 PO 4,
P 2 O 5 + 3CaO 2Ca 3 (PO 4) 2;
can form three types of salts:

Oxidizing properties are not typical for it, because The oxidation state +5 is very stable for phosphorus. Phosphorus pentoxide is obtained by burning phosphorus in a sufficient amount of oxygen:
4P + 5O 2 2P 2 O 5 .
Orthophosphoric acid H 3 PO 4 is a colorless crystalline substance, very soluble in water, hygroscopic. It is a triprotic acid of medium strength; does not have pronounced oxidizing properties. It exhibits all the chemical properties characteristic of acids and forms three types of salts (phosphates, hydrogen phosphates and dihydrogen phosphates):
2H 3 PO 4 + 3Ca = Ca 3 (PO 4) 2 + 3H 2,
H3PO4+Cu,
2H 3 PO 4 + 3CaO = Ca 3 (PO 4) 2 + 3H 2 O,

2H 3 PO 4 + K 2 CO 3 = 2KH 2 PO 4 + CO 2 + H 2 O.
In industry, phosphoric acid is obtained by extraction:
Ca 3 (PO 4) 2 + 3H 2 SO 4 = 2H 3 PO 4 + 3CaSO 4,
and also by the thermal method:
Ca 3 (PO 4) 2 + 3SiO 2 + 5C 3СaSiO 3 + 2P + 5CO,
4P + 5O 2 2P 2 O 5,
P 2 O 5 + 3H 2 O = 2H 3 PO 4.
Laboratory methods for obtaining orthophosphoric acid include the effect of dilute nitric acid on phosphorus:
3P (cr.) + 5HNO 3 (dil.) + 2H 2 O = 3H 3 PO 4 + 5NO,
interaction of metaphosphoric acid with water when heated:
HPO 3 + H 2 O H 3 PO 4 .
In the human body, orthophosphoric acid is formed during the hydrolysis of adenosinotriphosphoric acid (ATP):
ATP ADP + H 3 PO 4 .
Qualitative reaction to phosphate ion is a reaction with a silver cation; A yellow precipitate is formed, insoluble in slightly acidic media:
3Ag + + = Ag 3 PO 4,
3AgNO3 + K3PO4 = Ag3PO4 + 3KNO3.
In addition to the above phosphoric acids (containing phosphorus in the oxidation state +5), many other oxygen-containing acids are known for phosphorus. Here are some of the most important representatives.
Phosphorous(HPO 2 H 2) is a monobasic acid of medium strength. Its second name is phosphine:

Salts of this acid are called hypophosphites, or phosphites, for example KPO 2 H 2.
Phosphorous(H 3 PO 3) is a dibasic acid of medium strength, slightly weaker than hypophosphorous. It also has a second name – phosphonic:

Its salts are called phosphites, or phosphonates, for example K 2 PO 3 H.
Diphosphoric (pyrophosphoric)(H 4 P 2 O 7) - a tetrabasic acid of medium strength, slightly stronger than phosphoric acid:

Salts are diphosphates, for example K 4 P 2 O 7.
Test on the topic “Phosphorus and its compounds”
1. Eliminate the “extra” element from those listed according to the principle of the possibility of forming allotropic modifications:
a) oxygen; b) nitrogen;
c) phosphorus; d) sulfur.
2. When 42.6 g of phosphoric anhydride and 400 g of 15% sodium hydroxide solution react, the following is formed:
a) sodium phosphate;
b) sodium hydrogen phosphate;
c) a mixture of phosphate and sodium hydrogen phosphate;
d) a mixture of sodium hydro- and dihydrogen phosphate.
3. The sum of the coefficients in the equation for the electrolytic dissociation of potassium phosphate is equal to:
a) 5; b) 3; at 4; d) 8.
4. Number of electrons in the outer level of a phosphorus atom:
a) 2; b) 3; at 5; d) 15.
5. Phosphorus obtained from 33 g of technical calcium phosphate was burned in oxygen. The resulting phosphorus(V) oxide reacted with 200 ml of a 10% sodium hydroxide solution (density 1.2 g/ml) to form a medium salt. The mass of impurities in a technical sample of calcium phosphate (in g) is:
a) 3.5; b) 1.5; at 2; d) 4.8.
6. Number of -bonds in a pyrophosphoric acid molecule:
a) 2; b) 12; c) 14; d) 10.
7. The number of hydrogen atoms contained in 4.48 liters (n.s.) of phosphine is:
a) 1.2 10 23; b) 0.6 10 23;
c) 6.02 10 23; d) 3.6 10 23 .
8. At a temperature of 30 °C, a certain reaction takes place in 15 s, and at 0 °C - in 2 minutes. Van't Hoff coefficient for this reaction:
a) 2.4; b) 2; c) 1.8; d) 3.
9. Phosphoric acid can react with the following substances:
a) copper(II) oxide; b) potassium hydroxide;
c) nitric acid; d) zinc.
10. The sum of the coefficients in the reaction between phosphorus and Berthollet salt is equal to:
a) 9; b) 6; c) 19; d) such a reaction is impossible.
Key to the test
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| b | V | A | V | V | b | G | b | a, b, d | V |
Tasks and exercises on phosphorus and its compounds
Chain of transformations:
1. Phosphorus -> phosphorus pentoxide -> orthophosphoric acid -> calcium phosphate ® phosphoric acid.
2. Calcium phosphate -> phosphorus -> calcium phosphide -> phosphine -> phosphorus pentoxide -> phosphoric acid -> calcium dihydrogen phosphate.
3. Calcium phosphate -> A -> B -> C -> D -> E -> calcium phosphate. All substances contain phosphorus, in the scheme there are three ORPs in a row.
4. Phosphorus -> phosphorus pentoxide -> calcium phosphate -> phosphorus -> phosphine -> phosphoric acid -> calcium dihydrogen phosphate.
5. Calcium phosphide (+ hydrochloric acid solution) -> A (+ oxygen) -> B (+ sodium hydroxide, deficiency) -> C (+ sodium hydroxide, excess) -> D (+ calcium hydroxide) -> E.
| Level A |
1. With complete combustion of 6.8 g of the substance, 14.2 g of phosphorus pentoxide and 5.4 g of water were obtained. 37 ml of a 32% sodium hydroxide solution (density 1.35 g/ml) was added to the resulting reaction products. Establish the formula of the starting substance and determine the concentration of the resulting solution.
Solution
Reaction equation:
![]()
(P 2 O 5) = 0.1 mol, (H 2 O) = 0.3 mol.
(P) = 0.2 mol, (H) = 0.6 mol.
m(P) = 6.2 g, m(H) = 0.6 g.
m= 6.8 g.
(P) : (H) = 0.2: 0.6 = 1: 3.
Therefore, the formula of the starting substance is PH 3, and the reaction equation is:

then phosphoric acid is formed:
(H 3 PO 4) = 2(P 2 O 5) = 0.2 mol.
Phosphoric acid can react with alkali as follows:

Let us determine the amount of substance NaOH according to the conditions of the problem:

(H 3 PO 4) : (NaOH) = 0.2: 0.4 = 1: 2,
Therefore, reaction 2 occurs.
(Na 2 HPO 4) = (H 3 PO 4) = 0.2 mol;
m(Na 2 HPO 4) = M(Na 2 HPO 4) (Na 2 HPO 4) = 142 0.2 = 28.4 g;
m(r-ra) = m(P 2 O 5) + m(H 2 O) + m(NaOH solution) = 14.2 + 5.4 + 37 1.35 = 69.55 g.
(Na 2 HPO 4) = m(Na2HPO4)/ m(r-ra) = 28.4/69.55 = 0.4083, or 40.83%.
Answer. pH 3; (Na 2 HPO 4) = 40.83%.
2. With complete electrolysis of 1 kg of iron(II) sulfate solution, 56 g of metal was released at the cathode. What mass of phosphorus can react with the substance released at the anode, and what will be the composition of the salt if the resulting reaction product is dissolved in 87.24 ml of a 28% sodium hydroxide solution (solution density 1.31 g/ml)?
Answer. 12.4 g phosphorus; sodium hydrogen phosphate.
3. 20 g of a mixture consisting of barium sulfate, calcium phosphate, calcium carbonate and sodium phosphate were dissolved in water. The mass of the insoluble part was 18 g. When hydrochloric acid acted on it, 2.24 liters of gas (n.s.) were released and the mass of the insoluble residue was 3 g. Determine the composition of the initial mixture of salts by mass.
Answer. Na 3 PO 4 – 2 g; BaCO 3 – 3 g;
CaCO 3 – 10 g; Ca 3 (PO 4) 3 – 5 g.
4. How many kg of phosphorus can be obtained from 1 ton of phosphorite containing 40% impurities? What is the volume at standard conditions? will the phosphine obtained from this phosphorus take?
Answer. 120 kg P; 86.7 m 3 PH 3 .
5. 40 g of a mineral containing 77.5% calcium phosphate was mixed with excess sand and coal and heated without air in an electric furnace. The resulting simple substance was dissolved in 140 g of 90% nitric acid. Determine the mass of sodium hydroxide that will be required to completely neutralize the oxidation product of a simple substance.
Answer. 24 g NaOH.
| Level B |
1. To completely neutralize the solution obtained by hydrolysis of 1.23 g of some phosphorus halide, 35 ml of a 2M potassium hydroxide solution was required. Determine the formula of the halide.
Answer. Phosphorus trifluoride.
2. A sample of anhydrous ethanol containing 0.5% phosphorus(V) oxide as an impurity was burned in a sufficient amount of oxygen. The resulting gases were separated, and the resulting solution was heated until gas evolution stopped, after which an equal weight 0.5% solution of potassium hydroxide was added to it. Determine the mass fractions of substances in the resulting solution.
Answer. K 2 HPO 4 – 0.261%;
KH 2 PO 4 – 0.204%.
3. To 2 g of a mixture of hydrogen phosphate and potassium dihydrogen phosphate, in which the mass fraction of phosphorus is 20%, was added 20 g of a 2% solution of phosphoric acid. Calculate the mass fractions of substances in the resulting solution.
Answer. KH 2 PO 4 – 9.03%;
K 2 HPO 4 (remaining) – 1.87%.
4. When a mixture of alkali metal hydride and phosphide with equal mass fractions was treated with water, a gas mixture with a nitrogen density of 0.2926 was formed. Determine what metal was included in the compounds.
Answer. Sodium.
5. 50 g of a mixture of calcium phosphate and calcium and ammonium carbonates was calcined, resulting in 25.2 g of a solid residue, to which water was added, and then excess carbon dioxide was passed through. The mass of the undissolved residue was 14 g. Determine the mass of ammonium carbonate in the original mixture.
Solution
When the mixture is calcined, the following processes occur:
1) Ca 3 (PO 4) 2;
2) ![]()
3) (NH 4) 2 CO 3 2NH 3 + CO 2 + H 2 O.
The solid residue contains Ca 3 (PO 4) 2 and CaO.
After adding water:
4) Ca 3 (PO 4) 2 + H 2 O;
5) CaO + H 2 O = Ca(OH) 2.
After passing carbon dioxide:
6) Ca(OH) 2 + H 2 O + CO 2 = Ca(HCO 3) 2.
The undissolved residue is Ca 3 (PO 4) 2, therefore, m(Ca 3 (PO 4) 2) = 14 g.
Finding the mass of CaO:
m(CaO) = 25.2 – 14 = 11.2 g.
(CaO) = 11.2/56 = 0.2 mol,
(CaCO 3) = (CaO) = 0.2 mol,
m(CaCO 3) = 0.2 100 = 20 g.
m(NH 4) 2 CO 3 = m(mixtures) – m(Ca 3 (PO 4) 2) – m(CaCO 3) = 50 – 14 – 20 = 16 g.
Answer. m(NH 4) 2 CO 3 = 16 g.
QUALITATIVE TASKS
1. Solid, white, highly soluble in water, compound A is an acid. When oxide B is added to aqueous solution A, a white, water-insoluble compound C is formed. As a result of calcination of substance C at high temperature in the presence of sand and coal, a simple substance is formed, which is part of A. Identify the substances, write the reaction equations.
Answer. Substances: A – H 2 PO 4, B – CaO,
C – Ca 3 (PO 4) 2.
2. A mixture of two solids, red (A) and white (B), ignites with slight friction. The reaction produces two white solids, one of which (C) dissolves in water to form an acidic solution. If calcium oxide is added to substance C, a white, water-insoluble compound is formed. Identify substances, write reaction equations.
Answer. Substances: A – P (cr.), B – KClO 3,
C – P 2 O 5 .
3. The water-insoluble white compound A, as a result of calcination at high temperatures with coal and sand in the absence of oxygen, forms a simple substance B, which exists in several allotropic modifications. When substance B is burned, compound C is formed, which dissolves in water to form acid E, which is capable of forming three types of salts. Identify substances, write reaction equations.
Answer. Substances: A – Ca 3 (PO 4) 2, B – P,
C – P 2 O 5, E – H 3 PO 4.
* The +/– sign means that this reaction does not occur with all reagents or under specific conditions.
** An interesting one is the oxidation-reduction reaction (ORR) that occurs when matches are lit:
To be continued
Phosphine formula………………………………………………………….....PH 3
Molecular weight………………………………………………………34.04
Color and appearance................................................... .......Colorless gas.
Melting point................................... - 133.5 °C.
Boiling temperature................................................ .... -87.7°C.
Evaporation pressure........................40 mm Hg. Art. at - 129.4°C.
Solubility in water........................26% by volume at 17°C.
Density........................1.18 (0°C, 760 mmHg) (Air-1).
Flash point................................................... .....100°C.
Lower explosive limit........... 1.79-1.89% of volume;
Odor appears when................................................... ......1.3 - 2.6 ppm.
At relatively high concentrations, phosphine is explosive.
Lower flammability limit (LCFL) – 1.79-1.89%
by volume or ……………………………..26.15-27.60 g/m 3, or 17000-18900 ml/m 3.
The latent heat of evaporation of phosphine is …………………………………102.6 cal/g.
Solubility in water is 0.52 g/l at a temperature of 20 0 C and a pressure of 34.2 kgf/cm 2.
Phosphine
– a highly toxic, colorless gas that is 1.5 times heavier than air, therefore, when used, it easily penetrates into all cracks and hard-to-reach places in the premises and effectively destroys eggs, larvae, pupae and adult insects.
It dissolves poorly in water and does not react with it. Soluble in benzene, diethyl ether, carbon disulfide. Phosphine is highly toxic, affects the nervous system, and disrupts metabolism. MPC = 0.1 mg/m³. The odor is noticeable at a concentration of 2-4 mg/m³; prolonged inhalation at a concentration of 10 mg/m³ is fatal.
Application of phosphine. When carrying out fumigation with phosphine, inorganic preparations based on aluminum and magnesium phosphides are used. The objects and technology for using preparations based on magnesium phosphide are identical to those based on aluminum phosphide. Admission of people and loading of warehouses is permitted after complete ventilation and if the phosphine content in the air of the working area is not higher than the maximum permissible concentration (0.1 mg/m³). Products are sold with a phosphine residue not higher than the MRL (0.1 mg/kg for grain, 0.01 mg/kg for grain processing products).
Phosphine gas is a strong poison for humans and other warm-blooded animals. Acute phosphine poisoning occurs at a concentration in the air of 568 mg/m3. Phosphine gas is highly toxic to insects that pest grain stocks. When working with it, it is advisable to have an understanding of method and mechanism of action on harmful organisms. The maximum permissible concentration (MPC) of phosphine in the air of the working area is 0.1 mg/m3. However, the smell of gas begins to be felt at lower concentrations (about 0.03 mg/m3). The maximum permissible level (ML) of phosphine in grain is 0.01 mg/kg; phosphine residues are not allowed in grain products. Grain and products of its processing can be used for food purposes only if the residual amounts of phosphine in them do not exceed the MRL.
Phosphine gas It is weakly sorbed by grain and grain products, therefore it is easily degassed. At the consumption rates recommended for disinsection, it does not change the quality of the grain and does not impair its seed properties. It was first used in 1934 for the fumigation of grain products. Currently, due to the ban on the use of methyl bromide for fumigation purposes, phosphine is the main fumigant used to control harmful insects.
Phosphorus(from Greek phosphoros - luminiferous; lat. Phosphorus) P, chemical element of group V of the periodic system; atomic number 15, atomic mass 30.97376. It has one stable nuclide 31 P. The effective cross section for capturing thermal neutrons is 18 10 -30 m 2. External configuration electron shell of atom3 s 2 3p 3 ; oxidation states -3, +3 and +5; energy of sequential ionization during the transition from P 0 to P 5+ (eV): 10.486, 19.76, 30.163, 51.36, 65.02; electron affinity 0.6 eV; Pauling electronegativity 2.10; atomic radius 0.134 nm, ionic radii (coordination numbers are indicated in parentheses) 0.186 nm for P 3-, 0.044 nm (6) for P 3+, 0.017 nm (4 ), 0.029 nm (5), 0.038 nm (6) for P 5+ .
The average phosphorus content in the earth's crust is 0.105% by mass, in waters and oceans 0.07 mg/l. About 200 phosphorus minerals are known. they are all phosphates. Of these, the most important is apatite, which is the basis phosphorites. Also of practical importance are monazite CePO 4 , xenotime YPO 4 , amblygonite LiAlPO 4 (F, OH), triphylline Li(Fe, Mn)PO 4 , torbernite Cu(UO 2) 2 (PO 4) 2 12H 2 O, utunite Ca( UO 2) 2 (PO 4) 2 x x 10H 2 O, vivianite Fe 3 (PO 4) 2 8H 2 O, pyromorphite Pb 5 (PO 4) 3 C1, turquoise CuA1 6 (PO 4) 4 (OH) 8 5H 2 ABOUT.
Properties. It is known that St. 10 modifications of phosphorus, the most important of which are white, red and black phosphorus (technical white phosphorus is called yellow phosphorus). There is no uniform designation system for phosphorus modifications. Some properties of the most important modifications are compared in Table. Crystalline black phosphorus (PI) is thermodynamically stable under normal conditions. White and red phosphorus are metastable, but due to the low rate of transformation they can be preserved for an almost unlimited time under normal conditions.
Phosphorus compounds with nonmetals
Phosphorus and hydrogen in the form of simple substances practically do not interact. Hydrogen derivatives of phosphorus are obtained indirectly, for example:
Ca 3 P 2 + 6HCl = 3CaCl 2 + 2PH 3
Phosphine PH 3 is a colorless, highly toxic gas with the smell of rotten fish. A phosphine molecule can be thought of as an ammonia molecule. However, the angle between the H-P-H bonds is much smaller than that of ammonia. This means a decrease in the share of participation of s-clouds in the formation of hybrid bonds in the case of phosphine. Phosphorus-hydrogen bonds are less strong than nitrogen-hydrogen bonds. The donor properties of phosphine are less pronounced than those of ammonia. The low polarity of the phosphine molecule and weak proton-accepting activity lead to the absence of hydrogen bonds not only in liquid and solid states, but also with water molecules in solutions, as well as to the low stability of the phosphonium ion PH 4 +. The most stable phosphonium salt in the solid state is its iodide PH 4 I. Phosphonium salts vigorously decompose with water and especially alkaline solutions:
PH 4 I + KOH = PH 3 + KI + H 2 O
Phosphine and phosphonium salts are strong reducing agents. In air, phosphine burns to phosphoric acid:
PH 3 + 2O 2 = H 3 PO 4
When phosphides of active metals are decomposed by acids, diphosphine P 2 H 4 is formed simultaneously with phosphine as an impurity. Diphosphine is a colorless volatile liquid, similar in molecular structure to hydrazine, but phosphine does not exhibit basic properties. It ignites spontaneously in air and decomposes when stored in light or when heated. Its breakdown products contain phosphorus, phosphine and a yellow amorphous substance. This product is called solid hydrogen phosphide, and the formula P 12 H 6 is assigned to it.
With halogens, phosphorus forms tri- and pentahalides. These phosphorus derivatives are known for all analogues, but chlorine compounds are practically important. RG 3 and RG 5 are toxic and are obtained directly from simple substances.
RG 3 - stable exothermic compounds; PF 3 is a colorless gas, PCl 3 and PBr 3 are colorless liquids, and PI 3 are red crystals. In the solid state, all trihalides form crystals with a molecular structure. RG 3 and RG 5 are acid-forming compounds:
PI 3 + 3H 2 O = 3HI + H 3 PO 3
Both phosphorus nitrides are known, corresponding to the tri- and pentacovalent states: PN and P 2 N 5 . In both compounds, nitrogen is trivalent. Both nitrides are chemically inert and resistant to water, acids and alkalis.
Molten phosphorus dissolves sulfur well, but the chemical reaction occurs at high temperatures. Of the phosphorus sulfides, P 4 S 3 , P 4 S 7 , and P 4 S 10 are the best studied. These sulfides can be recrystallized in a naphthalene melt and isolated in the form of yellow crystals. When heated, sulfides ignite and burn to form P 2 O 5 and SO 2 . With water they all slowly decompose with the release of hydrogen sulfide and the formation of phosphorus oxygen acids.
Phosphorus compounds with metals
With active metals, phosphorus forms salt-like phosphides, which obey the rules of classical valence. p-Metals, as well as metals of the zinc subgroup, give both normal and anion-rich phosphides. Most of these compounds exhibit semiconductor properties, i.e. the dominant bond in them is covalent. The difference between nitrogen and phosphorus, due to size and energy factors, is most characteristically manifested in the interaction of these elements with transition metals. For nitrogen, when interacting with the latter, the main thing is the formation of metal-like nitrides. Phosphorus also forms metal-like phosphides. Many phosphides, especially those with predominantly covalent bonds, are refractory. Thus, AlP melts at 2197 degrees C, and gallium phosphide has a melting point of 1577 degrees C. Phosphides of alkali and alkaline earth metals are easily decomposed by water, releasing phosphine. Many phosphides are not only semiconductors (AlP, GaP, InP), but also ferromagnets, for example CoP and Fe 3 P.
Phosphine(hydrogen phosphide, phosphorus hydride, according to the IUPAC nomenclature - phosphane PH 3) - a colorless, very toxic, rather unstable gas with a specific smell of rotten fish.
Colorless gas. It dissolves poorly in water and does not react with it. At low temperatures it forms a solid clathrate 8РН 3 ·46Н 2 О. Soluble in benzene, diethyl ether, carbon disulfide. At −133.8 °C it forms crystals with a face-centered cubic lattice.
The phosphine molecule has the shape of a trigonal pyramid with molecular symmetry C 3v (d PH = 0.142 nm, HPH = 93.5 o). The dipole moment is 0.58 D, significantly lower than that of ammonia. The hydrogen bond between PH 3 molecules is practically not observed and therefore phosphine has lower melting and boiling points.
Phosphine is very different from its counterpart ammonia. Its chemical activity is higher than that of ammonia; it is poorly soluble in water, as a base is much weaker than ammonia. The latter is explained by the fact that the H-P bonds are weakly polarized and the activity of the lone pair of electrons in phosphorus (3s 2) is lower than that of nitrogen (2s 2) in ammonia.
In the absence of oxygen, when heated, it decomposes into elements:
spontaneously ignites in air (in the presence of diphosphine vapor or at temperatures above 100 °C):
Shows strong restorative properties:
When interacting with strong proton donors, phosphine can produce phosphonium salts containing the PH 4 + ion (similar to ammonium). Phosphonium salts, colorless crystalline substances, are extremely unstable and easily hydrolyze.
Like phosphine itself, its salts are strong reducing agents.
Phosphine is obtained by reacting white phosphorus with hot alkali, for example:
It can also be obtained by treating phosphides with water or acids:
Synthesis directly from elements is possible:
When heated, hydrogen chloride reacts with white phosphorus:
Decomposition of phosphonium iodide:
Decomposition of phosphonic acid:
or its restoration.