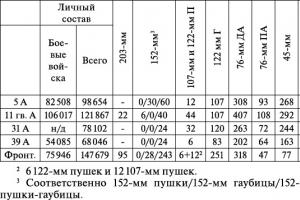
Oxygen. Oxygen and its properties. Topic: "Oxygen". The volume of air in the room. Methods for obtaining oxygen. Reactive oxygen species. Presentation on the topic: oxygen. Use of oxygen. This Air is Invisible. Allotropy of oxygen. Application of polymer materials. Oxygen in human life. Oxygen and its effect on the body.
Clean air is the key to health. General characteristics of the elements of the oxygen subgroup. Application of Biogas plants. Application of electronic educational resources in the educational process. Application of heat accumulators. Lesson topic: “Chemical properties of oxygen. In the format of training rooms. Oxygen is friend or foe. The children's room is the territory of happiness.
Thermite and propane-oxygen welding. Oxygen production, concept of catalysts. This substance is second only to oxygen in importance for human life. Oxygen 7th grade. Chemistry 7th grade oxygen. Ready-made movement therapy. Chemical properties of oxygen. The use of oxygen and its biological role.
Resources for oxygen and complex life support. Oxygen. Ozone is an allotropic modification of oxygen. Basic technological requirements for cladding gypsum plasterboard. How to obtain more and cheaper oxygen in a school laboratory.
1 slide
The presentation was prepared by Roxana Smirnova, a 9th grade student at the Lyceum of Otradnoye.

2 slide
Oxygen as an element. 1. The element oxygen is in group VI, main subgroup, period II, serial number No. 8, 2. Atomic structure: P11 = 8; n01 = 8; ē = 8 valency II, oxidation state -2 (rarely +2; +1; -1). 3. Part of oxides, bases, salts, acids, organic substances, including living organisms - up to 65% by weight.

3 slide
Oxygen as an element. Oxygen is the most common element on our planet. By weight, it accounts for approximately half of the total mass of all elements of the earth's crust. Air composition: O2 – 20-21%; N2 – 78%; CO2 – 0.03%, the rest comes from inert gases, water vapor, and impurities. 4. In the earth's crust it is 49% by mass, in the hydrosphere - 89% by mass. 5. Composed of air (in the form of a simple substance) – 20-21% by volume. 6. Included in most minerals and rocks (sand, clay, etc.). Composed of air (in the form of a simple substance). 7. A vital element for all organisms, found in most organic substances, involved in many biochemical processes that ensure the development and functioning of life. 8. Oxygen was discovered in 1769-1771. Swedish chemist K.-V. Scheele

4 slide
Physical properties. Oxygen is a chemically active non-metal and is the lightest element from the group of chalcogens. The simple substance oxygen under normal conditions is a colorless, tasteless and odorless gas, the molecule of which consists of two oxygen atoms, for which reason it is also called dioxygen. Liquid oxygen is light blue in color, while solid oxygen is light blue crystals.

5 slide
Chemical properties. With non-metals C + O2 CO2 S + O2 SO2 2H2 + O2 2H2O With complex substances 4FeS2 + 11O2 2Fe2O3 + 8SO2 2H2S + 3O2 2SO2 + 2H2O CH4 + 2O2 CO2 + 2H2O With metals 2Mg + O2 2MgO 2Cu + O2 –t 2CuO Interaction of substances with oxygen is called oxidation. All elements react with oxygen except Au, Pt, He, Ne and Ar; in all reactions (except for the interaction with fluorine), oxygen is an oxidizing agent. 1. Unstable: O3 O2 + O 2. Strong oxidizing agent: 2KI + O3 + H2O 2KOH + I2 + O2 Discolors dyes, reflects UV rays, destroys microorganisms.

6 slide
Methods of obtaining. Industrial method (distillation of liquid air). Laboratory method (decomposition of some oxygen-containing substances) 2KClO3 –t ;MnO2 2KCl + 3O2 2H2O2 –MnO2 2H2O + O2

7 slide
Checking the collected oxygen. Obtaining 3O2 2O3 During a thunderstorm (in nature), (in the laboratory) in an ozonizer of potassium permanganate when heated: 2KMnO4 –t K2MnO4 + MnO2 + O2 The decomposition of this salt occurs when it is heated above 2000 C.

8 slide
Applications of oxygen: It is widely used in medicine and industry. During high-altitude flights, pilots are provided with special oxygen devices. For many pulmonary and heart diseases, as well as during operations, oxygen is given to inhale from oxygen cushions. Submarines are supplied with oxygen in cylinders. The combustion of loose combustible material impregnated with liquid oxygen is accompanied by an explosion, which makes it possible to use oxygen in blasting operations. Liquid oxygen is used in jet engines, in autogenous welding and metal cutting, even under water.
1. History of the discovery of oxygen 2. The meaning of oxygen 3. Oxygen as an element 4. Oxygen as a simple substance 5. Physical properties of oxygen 6. Chemical properties 7. Methods of production 8. Applications of oxygen
Experiments from 1768 to 1773: “Research on air is currently the most important subject of chemistry” year: “Atmospheric air consists of two parts: “fiery air” - supports breathing and combustion, “spoiled air” - does not support combustion.”





1. The element oxygen is in group VI, main subgroup, period II, serial number 8, Ar = Atomic structure: P 1 1 = 8; n 0 1 = 8; ē = 8 valency II, oxidation state -2 (rarely +2; +1; -1). 3. Part of oxides, bases, salts, acids, organic substances, including living organisms - up to 65% by weight

4. In the earth’s crust it is 49% by mass, in the hydrosphere – 89% by mass. 5. Composed of air (in the form of a simple substance) – 20-21% by volume. Air composition: O 2 –%; N 2 – 78%; CO 2 – 0.03%, the rest is inert gases, water vapor, impurities Oxygen is the most common element on our planet. By weight, it accounts for approximately half of the total mass of all elements of the earth's crust.


Chemical formula – O 2, Mr (O 2) = 32; M = 32 g/mol. The atmosphere contains about 21% oxygen (1/5 part). A person inhales approximately 750 liters of oxygen per day. The main suppliers of oxygen are tropical forests and ocean phytoplankton. Every year, as a result of photosynthesis, 3000 billion tons of oxygen enter the Earth's atmosphere.

Gas - colorless, tasteless and odorless; 3V O 2 (n.s.) dissolves in 100V H 2 O; t boil= -183 C; t pl = -219 C; d by air = 1.1. At a pressure of 760 mm. Hg and a temperature of –183 C, oxygen liquefies


With non-metals C + O 2 CO 2 S + O 2 SO 2 2H 2 + O 2 2H 2 O With complex substances 4FeS O 2 2Fe 2 O 3 + 8SO 2 2H 2 S + 3O 2 2SO 2 + 2H 2 O CH 4 + 2O 2 CO 2 + 2H 2 O With metals 2Mg + O 2 2MgO 2Cu + O 2 – t 2CuO The interaction of substances with oxygen is called oxidation. All elements react with oxygen except Au, Pt, He, Ne and Ar; in all reactions (except for the interaction with fluorine), oxygen is an oxidizing agent. Ozone gas – 1. Unstable: O 3 O 2 + O 2. Strong oxidizing agent: 2KI + O 3 + H 2 O 2KOH + I 2 + O 2 Discolors dyes, reflects UV rays, destroys microorganisms. Oxygen gas reacts:


I. Industrial method (distillation of liquid air). II. Laboratory method (decomposition of some oxygen-containing substances) 2KClO 3 – t;MnO2 2KCl + 3O 2 2H 2 O 2 – MnO2 2H 2 O + O 2 Obtaining 3O 2 2O 3 During a thunderstorm (in nature), (in the laboratory) in an ozonizer

Potassium permanganate when heated: 2KMnO 4 – t K 2 MnO 4 + MnO 2 + O 2 The decomposition of this salt occurs when it is heated above C. Heating 2KMnO 4 Checking the collected oxygen

Displacement of water displacement of air =

It is widely used in medicine and industry. During high-altitude flights, pilots are provided with special oxygen devices. For many pulmonary and heart diseases, as well as during operations, oxygen is given to inhale from oxygen cushions. Submarines are supplied with oxygen in cylinders. The combustion of loose combustible material impregnated with liquid oxygen is accompanied by an explosion, which makes it possible to use oxygen in blasting operations. Liquid oxygen is used in jet engines, in autogenous welding and metal cutting, even under water

1.Who called oxygen “fiery” and nitrogen “spoilt” air? A) Lavoisier B) Priestley C) Scheele 2. What substances does the chemical element oxygen form? A) only simple substances B) simple and complex substances C) only complex substances. 3.What are the names of binary compounds whose molecules are formed by atoms of a chemical element and oxygen: A) sulfides, B) chlorides, C) oxides

4. In 1774, one scientist, after an experiment, wrote: “But what struck me most of all was that the candle burned in this air with an amazingly brilliant flame...” It was: A) Lavoisier B) Priestley C) Scheele 5. Title “ Oxygenium" suggested: A) Lavoisier B) Priestley C) Scheele 6. Oxygen in water: A) highly soluble B) slightly soluble C) does not dissolve at all 7. When oxygen is blown into a flame, the temperature of the flame: A) does not change B) decreases C) rises

8. Iron (III) oxide has the formula: A) Fe 2 O 3 B) FeO 3 C) Fe 3 O 4 9. In which equation are the coefficients correct: A) 2P + O 2 = P 2 O 5 B) 2P + 5O 2 = P 2 O 5 C) 4P + 5O 2 = 2P 2 O 5 10. In which row are all three formulas written correctly: A) P 2 O 5, Al 2 O, H 2 O B) MgO, Al 2 O 3, CO 2 C) CO 2, HO, P 2 O 5

Slide 1
 Slide 2
Slide 2
 Slide 3
Slide 3
 Slide 4
Slide 4
 Slide 5
Slide 5
 Slide 6
Slide 6
 Slide 7
Slide 7
 Slide 8
Slide 8
 Slide 9
Slide 9
The presentation on the topic "Oxygen" can be downloaded absolutely free on our website. Project subject: Chemistry. Colorful slides and illustrations will help you engage your classmates or audience. To view the content, use the player, or if you want to download the report, click on the corresponding text under the player. The presentation contains 9 slide(s).
Presentation slides

Slide 1
Oxygen O2
12.11.10. Anfisa Antipovna Kuptsova, teacher of chemistry and biology, Shatrakasinsky secondary school, Morgaushsky district, Chuvash Republic

Slide 2
Oxygen as an element.
1. The element oxygen is in group VI, main subgroup, period II, serial number No. 8, Ar = 16. 2. Atomic structure: P11 = 8; n01 = 8; ē = 8 valency II, oxidation state -2 (rarely +2; +1; -1). 3. Part of oxides, bases, salts, acids, organic substances, including living organisms - up to 65% by weight.

Slide 3
Oxygen as an element (continued).
4. In the earth's crust it is 49% by mass, in the hydrosphere - 89% by mass. 5. Composed of air (in the form of a simple substance) – 20-21% by volume. Air composition: O2 – 20-21%; N2 – 78%; CO2 – 0.03%, the rest comes from inert gases, water vapor, and impurities.
Oxygen is the most common element on our planet. By weight, it accounts for approximately half of the total mass of all elements of the earth's crust.

Slide 4
Physical properties
Gas - colorless, tasteless and odorless; 3V O2 (n.s.) dissolves in 100V H2O; tboiling= -183С; tpl = -219C; d by air = 1.1. At a pressure of 760 mm. Hg and a temperature of –183 С, oxygen liquefies

Slide 5
Chemical properties
With non-metals C + O2 CO2 S + O2 SO2 2H2 + O2 2H2O
With complex substances 4FeS2 + 11O2 2Fe2O3 + 8SO2 2H2S + 3O2 2SO2 + 2H2O CH4 + 2O2 CO2 + 2H2O
With metals 2Mg + O2 2MgO 2Cu + O2 –t 2CuO
The interaction of substances with oxygen is called oxidation. All elements react with oxygen except Au, Pt, He, Ne and Ar; in all reactions (except for the interaction with fluorine), oxygen is an oxidizing agent. 1. Unstable: O3 O2 + O 2. Strong oxidizing agent: 2KI + O3 + H2O 2KOH + I2 + O2 Discolors dyes, reflects UV rays, destroys microorganisms.

Slide 6
Methods of obtaining
Industrial method (distillation of liquid air). Laboratory method (decomposition of some oxygen-containing substances) 2KClO3 –t;MnO2 2KCl + 3O2 2H2O2 –MnO2 2H2O + O2 Obtaining 3O2 2O3 During a thunderstorm (in nature), (in the laboratory) in an ozonator

Slide 7
Methods for producing oxygen (continued).
potassium permanganate when heated: 2KMnO4 –t K2MnO4 + MnO2 + O2 Decomposition of this salt occurs when it is heated above 2000 C. Heating 2KMnO4 Checking the collected oxygen

Slide 8
Collection methods
displacement of water displacement of air =

Tips for making a good presentation or project report
- Try to involve the audience in the story, set up interaction with the audience using leading questions, a game part, do not be afraid to joke and smile sincerely (where appropriate).
- Try to explain the slide in your own words, add additional interesting facts; you don’t just need to read the information from the slides, the audience can read it themselves.
- There is no need to overload the slides of your project with text blocks; more illustrations and a minimum of text will better convey information and attract attention. The slide should contain only key information; the rest is best told to the audience orally.
- The text must be well readable, otherwise the audience will not be able to see the information being presented, will be greatly distracted from the story, trying to at least make out something, or will completely lose all interest. To do this, you need to choose the right font, taking into account where and how the presentation will be broadcast, and also choose the right combination of background and text.
- It is important to rehearse your report, think about how you will greet the audience, what you will say first, and how you will end the presentation. All comes with experience.
- Choose the right outfit, because... The speaker's clothing also plays a big role in the perception of his speech.
- Try to speak confidently, smoothly and coherently.
- Try to enjoy the performance, then you will be more at ease and less nervous.