Universal measure
An original proposal was once made by S. Pudlovsky, a professor at the University of Krakow. His idea was that as a single measure we should take the length of the pendulum that makes a full swing in one second. This proposal was published in the book “Universal Measure”, published in Vilna in 1675 by his student T. Buratini. He also suggested calling meter unit of length.
Somewhat earlier, in 1673, the Dutch scientist H. Huygens published a brilliant work “Pendulum Clocks”, where he developed the theory of oscillations and described the designs of pendulum clocks. Based on this work, Huygens proposed his own universal measure of length, which he called hour foot, and the hour foot was equal to 1/3 the length of the second pendulum. “This measure can not only be determined everywhere in the world, but can always be restored for all future centuries,” Huygens wrote proudly.
However, there was one circumstance that confused scientists. The period of oscillation of a pendulum with the same length was different depending on the geographic latitude, i.e., strictly speaking, the measure was not universal.
Huygens' idea was promoted by the French surveyor C. Condamine, who proposed to base the measurement system on a unit of length corresponding to the length of a pendulum swinging once per second at the equator.
The French astronomer and mathematician G. Mouton also supported the idea of a second pendulum, but only as a control device, and G. Mouton proposed to base the universal system of measures on the principle of connecting the unit of measurement with the dimensions of the Earth, i.e., taking part as a unit of length meridian arc length. This scientist also proposed dividing the measured part into tenths, hundredths and thousandths, i.e. using the decimal principle.
Metric system
Projects for reform of systems of measures appeared in different countries, but this issue was especially acute in France for the reasons listed above. Gradually, the idea of creating a system of measures that meets certain requirements emerged:
– the system of measures must be unified and general;
– units of measurement must have strictly defined dimensions;
– there must be standards of units of measurement that are constant over time;
– for each quantity there should be only one unit;
– units of different quantities must be related to each other in a convenient way;
– units must have submultiple and multiple values.
On May 8, 1790, the French National Assembly adopted a decree on the reform of the system of measures and instructed the Paris Academy of Sciences to carry out the necessary work, guided by the above requirements.
Several commissions were formed. One of them, led by academician Lagrange, recommended the decimal division of multiples and submultiples of units.
Another commission, which included scientists Laplace, Monge, Borda and Condors, proposed adopting one forty millionth of the earth's meridian as a unit of length, although the overwhelming majority of experts who knew the essence of the matter thought that the choice would be in favor of the second pendulum.
The decisive factor here was that a stable basis was chosen - the size of the Earth, the correctness and immutability of its shape in the form of a ball.
Commission member C. Borda, a surveyor and hydraulic engineer, proposed calling the unit of length the meter; in 1792 he determined the length of the second pendulum in Paris.
On March 26, 1791, the French National Assembly approved the proposal of the Paris Academy, and a temporary commission was formed to practically implement the decree on the reform of measures.
On April 7, 1795, the French National Convention adopted a law on new weights and measures. It was accepted that meter- one ten-millionth of a quarter of the earth's meridian passing through Paris. but it was especially emphasized that the introduced unit of length in name and size did not coincide with any of the French units of length that existed at that time. Therefore, the possible future argument that France is “pushing” its system of measures as an international one is excluded.
Instead of temporary commissions, commissioners were appointed who were tasked with carrying out work on the experimental determination of units of length and mass. The commissioners included famous scientists Berthollet, Borda, Brisson, Coulomb, Delambre, Haüy, Lagrange, Laplace, Mechain, Monge and others.
Delambre and Méchain resumed work on measuring the length of the meridian arc between Dunkirk and Barcelona, corresponding to the 9°40′ sphere (this arc was later extended from the Shetland Islands to Algeria).
This work was completed by the fall of 1798. Meter and kilogram standards were made of platinum. The meter standard was a platinum bar 1 meter long and with a cross-section of 25 × 4 mm, i.e. it was end measure, and on June 22, 1799, the ceremonial transfer of the prototypes of the meter and kilogram to the Archives of France took place, and since then they have been called archival. But it must be said that even in France the metric system was not established immediately; traditions and inertia of thinking had a significant impact. Napoleon, who became Emperor of France, did not like the metric system, to put it mildly. He believed: “There is nothing more contrary to the mindset, memory and consideration than what these scientists propose. The good of present generations has been sacrificed to abstractions and empty hopes, for in order to force the old nation to accept new units of weights and measures, it is necessary to redo all administrative rules, all industrial calculations. This kind of work boggles the mind.” In 1812, by decree of Napoleon, the metric system in France was abolished and only in 1840 was it restored again.
Gradually, the metric system was adopted and introduced by Belgium, Holland, Spain, Portugal, Italy, and a number of South American republics. The initiators of the introduction of the metric system in Russia were, of course, scientists, engineers, and researchers, but tailors, seamstresses and milliners played a significant role - by that time, Parisian fashion had conquered high society, and there, mostly craftsmen who came from abroad worked there with their own meters . It was from them that the narrow strips of oilcloth fabric that still exist today - “centimeters”, which are still used today, came from.
At the Paris Exhibition of 1867, the International Committee of Weights, Measures and Coins was created, which compiled a report on the benefits of the metric system. However, the decisive influence on the entire further course of events was exerted by the report compiled in 1869 by academicians O. V. Struve, G. I. Wild and B. S. Jacobi, sent on behalf of the St. Petersburg Academy of Sciences to the Paris Academy. The report argued for the need to introduce an international system of weights and measures based on the metric system.
The proposal was supported by the Paris Academy, and the French government appealed to all interested states with a request to send scientists to the International Metric Commission to solve practical problems. By that time, it became clear that the shape of the Earth is not a sphere, but a three-dimensional spheroid (the average radius of the equator is 6,378,245 meters, the difference between the largest and smallest radii is 213 meters, and the difference between the average radius of the equator and the polar semi-axis is 21,382 meters). In addition, repeated measurements of the arc of the Paris meridian gave a value of the meter slightly smaller compared to the value obtained by Delambre and Méchain. In addition, there is always the possibility that with the creation of more advanced measuring instruments and the emergence of new measurement methods, the measurement results will change. Therefore, the commission made an important decision: “The new prototype of the length measure should be equal in size to the Archival meter,” that is, it should be an artificial standard.
The international commission also made the following decisions.
1) The new prototype meter should be a line measure, it should be made of an alloy of platinum (90%) and iridium (10%) and have an X-shaped cross-section.
2) In order to give the metric system an international character and ensure uniformity of measures, standards should be produced and distributed among the countries concerned.
3) One standard, closest in size to the Archive, should be accepted as international.
4) Entrust practical work on creating standards to the French section of the commission, since archival prototypes are located in Paris.
5) Appoint a permanent international committee of 12 members to supervise the work.
6) Establish the International Bureau of Weights and Measures as a neutral scientific institution based in France.
In accordance with the decision of the commission, practical measures were carried out and in 1875 an international conference was convened in Paris, at the last meeting of which, on May 20, 1875, the Meter Convention was signed. It was signed by 17 countries: Austria-Hungary, Argentina, Belgium, Brazil, Venezuela, Germany, Denmark, Spain, Italy, France, Peru, Portugal, Russia, USA, Turkey, Switzerland, Sweden and Norway (as one country). Three more countries (Great Britain, Holland, Greece), although they participated in the conference, did not sign the Convention due to disagreement on the functions of the International Bureau.
The Bretel Pavilion, located in the Saint-Cloud park in the Paris suburb of Sevres, was allocated for the International Bureau of Weights and Measures; soon a laboratory building with equipment was built near this pavilion. The activities of the Bureau are carried out at the expense of funds transferred by the member countries of the Convention in proportion to the size of their population. Using these funds, standards for the meter and kilogram (36 and 43, respectively) were ordered in England, which were manufactured in 1889.
Meter standards
The meter standard was a platinum-iridium rod with an X-shaped cross-section, 1020 mm long. On a neutral plane at 0 °C, three strokes were applied on each side, the distance between the middle strokes was 1 meter (Fig. 1.1). The standards were numbered and compared with the Archive Meter. Prototype No. 6 turned out to be the closest to the Archive, and it was approved as an international prototype. Thus, the standard meter became artificial and represented lined measure.
Four more witness standards were added to standard No. 6 and these were retained by the International Bureau. The remaining standards were distributed by lot among the countries that signed the Convention. Russia received standards No. 11 and No. 28, and No. 28 was closer to the international prototype, so it became the national standard of Russia.
By decree of the Council of People's Commissars of the RSFSR dated September 11, 1918, prototype No. 28 was approved as the state primary standard of the meter. In 1925, the Council of People's Commissars of the USSR adopted a resolution recognizing the Metric Convention of 1875 as valid for the USSR.
In 1957 - 1958 standard No. 6 was marked with a scale with decimeter divisions, the first decimeter was divided into 10 centimeters, and the first centimeter into 10 millimeters. After applying the strokes, this standard was re-certified by the International Bureau of Weights and Measures.
The error in transmitting a unit of length from the standard to the measuring instruments was 0.1 - 0.2 microns, which with the development of technology is becoming clearly insufficient, therefore, in order to reduce the transmission error and obtain a natural indestructible standard, a new meter standard was created.
Back in 1829, the French physicist J. Babinet proposed taking the length of a certain line in the spectrum as a unit of length. However, the practical implementation of this idea occurred only when the American physicist A. Michelson invented the interferometer. Together with the chemist Morley E. Babinet, J. published the work “On the method of using the wavelength of sodium light as a natural and practical standard of length,” then he moved on to studies of isotopes: mercury - green and cadmium - red line.
In 1927, it was accepted that 1 m was equal to 1553164.13 wavelengths of the red line of cadmium-114, this value was accepted as a standard along with the old prototype meter.
Subsequently, work was continued: the spectrum of mercury was studied in the USA, the spectrum of cadmium was studied in the USSR, krypton was studied in Germany and France.
In 1960, the XI General Conference on Weights and Measures adopted the meter, expressed in wavelengths of light, specifically the inert gas Kr-86, as the standard unit of length. Thus, the standard of the meter again became natural.
Meter– length equal to 1650763.73 wavelengths in vacuum of radiation corresponding to the transition between levels 2p 10 and 5d 5 of the krypton-86 atom. The old definition of the meter is abolished, but the prototypes of the meter remain and are stored under the same conditions.
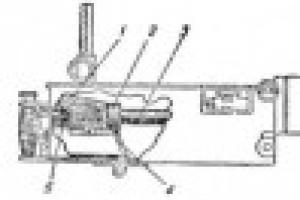
In accordance with this decision, the State Primary Standard (GOST 8.020-75) was established in the USSR, which included the following components (Fig. 1.2):
1) source of primary reference radiation of krypton-86;
2) a reference interferometer used to study sources of primary reference radiation;
The accuracy of reproduction and transmission of the meter in light units is 1∙10 -8 m.

In 1983, the XVII General Conference on Weights and Measures adopted a new definition of the meter: 1 meter is a unit of length equal to the path traveled by light in a vacuum in 1/299792458 of a second, i.e. the standard of the meter remains natural.
Composition of the meter standard:
1) source of primary reference radiation – a highly frequency-stabilized helium-neon laser;
2) a reference interferometer used to study sources of primary and secondary reference measurements;
3) a standard interferometer used to measure the length of line and end standards (secondary standards).
Oops... Javascript not found.
Sorry, JavaScript is disabled or not supported in your browser.
Unfortunately, this site will not work without JavaScript. Check your browser settings, maybe JavaScript is disabled by accident?
Metric system (SI International System)
Metric system of measures (SI International System)
For residents of the United States or another country that does not use the metric system, it is sometimes difficult to understand how the rest of the world lives in and navigates it. But in fact, the SI system is much simpler than all traditional national measurement systems.
The principles of the metric system are very simple.
The structure of the international system of SI units
The metric system was developed in France in the 18th century. The new system was intended to replace the chaotic collection of different units of measurement then in use with a single common standard with simple decimal coefficients.
The standard unit of length was defined as one ten-millionth of the distance from the Earth's north pole to the equator. The resulting value was called meter. The definition of meter was later refined several times. The modern and most accurate definition of a meter is: “the distance that light travels in a vacuum in 1/299,792,458 of a second.” Standards for the remaining measurements were established in a similar manner.
The metric system or International System of Units (SI) is based on seven basic units for seven basic dimensions, independent of each other. These measurements and units are: length (meter), mass (kilogram), time (second), electric current (ampere), thermodynamic temperature (kelvin), amount of substance (mole) and radiation intensity (candela). All other units are derived from the base ones.
All units of a specific measurement are built on the basis of the base unit by adding universal ones metric prefixes. A table of metric prefixes is shown below.
Metric prefixes
Metric prefixes simple and very convenient. It is not necessary to understand the nature of the unit in order to convert a value from, for example, kilo units to mega units. All metric prefixes are powers of 10. The most commonly used prefixes are highlighted in the table.
By the way, on the Fractions and Percentages page you can easily convert a value from one metric prefix to another.
| Prefix | Symbol | Degree | Factor |
|---|---|---|---|
| yotta | Y | 10 24 | 1,000,000,000,000,000,000,000,000 |
| zetta | Z | 10 21 | 1,000,000,000,000,000,000,000 |
| exa | E | 10 18 | 1,000,000,000,000,000,000 |
| peta | P | 10 15 | 1,000,000,000,000,000 |
| tera | T | 10 12 | 1,000,000,000,000 |
| giga | G | 10 9 | 1,000,000,000 |
| mega | M | 10 6 | 1,000,000 |
| kilo | k | 10 3 | 1,000 |
| hecto | h | 10 2 | 100 |
| soundboard | da | 10 1 | 10 |
| deci | d | 10 -1 | 0.1 |
| centi | c | 10 -2 | 0.01 |
| Milli | m | 10 -3 | 0.001 |
| micro | µ | 10 -6 | 0.000,001 |
| nano | n | 10 -9 | 0.000,000,001 |
| pico | p | 10 -12 | 0,000,000,000,001 |
| femto | f | 10 -15 | 0.000,000,000,000,001 |
| atto | a | 10 -18 | 0.000,000,000,000,000,001 |
| cepto | z | 10 -21 | 0.000,000,000,000,000,000,001 |
| yocto | y | 10 -24 | 0.000,000,000,000,000,000,000,001 |
Even in countries that use the metric system, most people only know the most common prefixes, such as kilo, milli, mega. These prefixes are highlighted in the table. The remaining prefixes are used mainly in science.
Back
History of the creation of the metric system


As you know, the metric system originated in France at the end of the 18th century. The variety of weights and measures, the standards of which sometimes differed significantly in different regions of the country, often led to confusion and conflict. Thus, there is an urgent need to reform the current measurement system or develop a new one, taking as a basis a simple and universal standard. In 1790, a project by the well-known Prince Talleyrand, who later became the Minister of Foreign Affairs of France, was submitted for discussion to the National Assembly. As a standard of length, the activist proposed to take the length of the second pendulum at a latitude of 45°.
By the way, the idea of a pendulum was no longer new at that time. Back in the 17th century, scientists made attempts to determine universal meters based on real objects that maintained a constant value. One of these studies belonged to the Dutch scientist Christiaan Huygens, who conducted experiments with a second pendulum and proved that its length depends on the latitude of the place where the experiment was carried out. A century before Talleyrand, based on his own experiments, Huygens proposed using 1/3 the length of a pendulum with a period of oscillation of 1 second, which was approximately 8 cm, as a global standard of length.
And yet, the proposal to calculate the standard of length using the readings of a second pendulum did not find support in the Academy of Sciences, and the future reform was based on the ideas of the astronomer Mouton, who calculated the unit of length from the arc of the earth's meridian. He also came up with a proposal to create a new measurement system on a decimal basis.
In his project, Talleyrand outlined in detail the procedure for determining and introducing a single standard of length. Firstly, it was supposed to collect all possible measures from all over the country and bring them to Paris. Secondly, the National Assembly was to contact the British Parliament with a proposal to create an international commission of leading scientists from both countries. After the experiment, the French Academy of Sciences had to establish the exact relationship between the new unit of length and the measures that had previously been used in various parts of the country. Copies of the standards and comparative tables with the old measures had to be sent to all regions of France. This regulation was approved by the National Assembly, and on August 22, 1790, it was approved by King Louis XVI.
Work on determining the meter began in 1792. The leaders of the expedition, which was tasked with measuring the meridian arc between Barcelona and Dunkirk, were the French scientists Mechain and Delambre. The work of French scientists was planned for several years. However, in 1793, the Academy of Sciences, which carried out the reform, was abolished, which caused a serious delay in the already difficult, labor-intensive research. It was decided not to wait for the final results of measuring the meridian arc and to calculate the length of the meter based on existing data. So in 1795, the temporary meter was defined as 1/10000000 of the Parisian meridian between the equator and the north pole. Work to clarify the meter was completed by the fall of 1798. The new meter was shorter by 0.486 lines or 0.04 French inches. It was this value that formed the basis of the new standard, legalized on December 10, 1799.
One of the main provisions of the metric system is the dependence of all measures on a single linear standard (meter). So, for example, when determining the basic unit of weight - - it was decided to take a cubic centimeter of pure water as a basis.
By the end of the 19th century, almost all of Europe, with the exception of Greece and England, had adopted the metric system. The rapid spread of this unique system of measures, which we still use today, was facilitated by simplicity, unity and accuracy. Despite all the advantages of the metric system, Russia at the turn of the 19th - 20th centuries did not dare to join the majority of European countries, even then breaking the centuries-old habits of the people and abandoning the use of the traditional Russian system of measures. However, the “Regulations on Weights and Measures” dated June 4, 1899 officially allowed the use of the kilogram along with the Russian pound. The final measurements were completed only by the beginning of the 1930s.
(15.II.1564 - 8.I.1642) - an outstanding Italian physicist and astronomer, one of the founders of exact natural science, member of the Accademia dei Lincei (1611). R. in Pisa. In 1581 he entered the University of Pisa, where he studied medicine. But, fascinated by geometry and mechanics, in particular the works of Archimedes and Euclid, he left the university with its scholastic lectures and returned to Florence, where he studied mathematics on his own for four years.
From 1589 - professor at the University of Pisa, in 1592 -1610 - at the University of Padua, later - court philosopher of Duke Cosimo II de' Medici.
He had a significant influence on the development of scientific thought. It is from him that physics as a science originates. Humanity owes Galileo two principles of mechanics, which played a large role in the development of not only mechanics, but also all physics. This is the well-known Galilean principle of relativity for rectilinear and uniform motion and the principle of constancy of the acceleration of gravity. Based on the Galilean principle of relativity, I. Newton came to the concept of an inertial frame of reference, and the second principle associated with the free fall of bodies led him to the concept of inertial and heavy mass. A. Einstein extended Galileo's mechanical principle of relativity to all physical processes, in particular to light, and derived from it consequences about the nature of space and time (in this case, Galileo's transformations were replaced by Lorentz transformations). The combination of the second Galilean principle, which Einstein interpreted as the principle of the equivalence of inertial forces to gravitational forces, with the principle of relativity led him to the general theory of relativity.
Galileo established the law of inertia (1609), the laws of free fall, the movement of a body on an inclined plane (1604 - 09) and a body thrown at an angle to the horizon, discovered the law of addition of movements and the law of constancy of the period of oscillation of a pendulum (the phenomenon of isochronism of oscillations, 1583). Dynamics originates from Galileo.
In July 1609, Galileo built his first telescope - an optical system consisting of a convex and concave lens - and began systematic astronomical observations. This was the rebirth of the telescope, which, after almost 20 years of obscurity, became a powerful tool of scientific knowledge. Therefore, Galileo can be considered the inventor of the first telescope. He quickly improved his telescope and, as he wrote over time, “built himself a device so wonderful that with its help objects seemed almost a thousand times larger and more than thirty times closer than when observed with a simple eye.” In his treatise “The Starry Messenger,” published in Venice on March 12, 1610, he described the discoveries made with the help of a telescope: the discovery of mountains on the Moon, four satellites of Jupiter, proof that the Milky Way consists of many stars.
The creation of the telescope and astronomical discoveries brought Galileo wide popularity. Soon he discovers the phases of Venus, spots on the Sun, etc. Galileo sets up the production of telescopes. By changing the distance between the lenses, 1610 -14 also creates a microscope. Thanks to Galileo, lenses and optical instruments became powerful tools for scientific research. As S.I. Vavilov noted, “it was from Galileo that optics received the greatest incentive for further theoretical and technical development.” Galileo's optical research was also devoted to the doctrine of color, questions of the nature of light, and physical optics. Galileo came up with the idea of the finiteness of the speed of propagation of light and setting up (1607) an experiment to determine it.
Galileo's astronomical discoveries played a huge role in the development of the scientific worldview; they clearly convinced of the correctness of the teachings of Copernicus, the fallacy of the system of Aristotle and Ptolemy, and contributed to the victory and establishment of the heliocentric system of the world. In 1632, the famous “Dialogue on the Two Chief Systems of the World” was published, in which Galileo defended the heliocentric system of Copernicus. The publication of the book enraged the clergy, the Inquisition accused Galileo of heresy and, having organized a trial, forced him to publicly renounce the Copernican teachings, and imposed a ban on the Dialogue. After the trial in 1633, Galileo was declared a “prisoner of the Holy Inquisition” and was forced to live first in Rome and then in Archertri near Florence. However, Galileo did not stop his scientific activity; before his illness (in 1637 Galileo finally lost his sight), he completed the work “Conversations and Mathematical Proofs Concerning Two New Branches of Science,” which summed up his physical research.
Invented the thermoscope, which is the prototype thermometer, designed (1586) hydrostatic scales to determine the specific gravity of solids, he determined the specific gravity of air. He put forward the idea of using a pendulum in a clock. Physical research is also devoted to hydrostatics, strength of materials, etc.
Blaise Pascal, concept of atmospheric pressure
(19.VI.1623 - 19.VIII.1662) - French mathematician, physicist and philosopher. R. in Clermont-Ferrand. Received home education. In 1631 he moved with his family to Paris. Mathematicians and physicists gathered every week at E. Pascal and some of his friends - M. Mersenne, J. Roberval and others. These meetings eventually turned into scientific ones. meetings. Paris was created on the basis of this circle. AN (1666). From the age of 16, P. took part in the work of the circle. At this time, he wrote his first work on conic sections, in which he stated one of the important theorems of projective geometry: the intersection points of opposite sides of a hexagon inscribed in a conic section lie on the same straight line (Pascal’s theorem).
Physical research relates mainly to hydrostatics, where in 1653 he formulated its basic law, according to which pressure on a liquid is transmitted evenly without change in all directions - Pascal's law (this property of a liquid was known to his predecessors), established the principle of operation of a hydraulic press. He rediscovered the hydrostatic paradox, which became widely known thanks to him. Confirmed existence atmospheric pressure, repeating Torricelli's experiment with water and wine in 1646. He expressed the idea that atmospheric pressure decreases with height (based on his idea, an experiment was carried out in 1647, which showed that at the top of a mountain the level of mercury in a tube is lower than at the base), demonstrated the elasticity of air, proved that air has weight, discovered that barometer readings depend on air humidity and temperature, and therefore it can be used to predict the weather.
In mathematics, he devoted a number of works to arithmetic series and binomial coefficients. In his “Treatise on the Arithmetic Triangle” he gave the so-called. Pascal's triangle - a table with coefficients. the expansions (a+b)n for different n are arranged in the form of a triangle. Binomial coefficients formed a complete mathematics according to the method he developed. induction - this was one of his most important discoveries. What was also new was that binomial coefficients. acted here as numbers of combinations of n elements by m and were then used in problems in probability theory. Until that time, no mathematician had calculated the probability of events. Pascal and P. Fermanagh found the key to solving such problems. In their correspondence, probability theory and combinatorics are scientifically substantiated, and therefore Pascal and Fermat are considered the founders of a new field of mathematics - probability theory. He also made a great contribution to the development of infinitesimal calculus. While studying the cycloid, he proposed general methods for determining quadratures and centers of gravity. curves, discovered and applied such methods, which give reason to consider him one of the creators of infinitesimal calculus. In his “Treatise on the Sines of the Quarter Circle,” when calculating the integrals of trigonometric functions, in particular the tangent, he introduced elliptic integrals, which later played an important role in analysis and its applications. In addition, he proved a number of theorems concerning changes of variables and integration by parts. Pascal contains, although in an undeveloped form, ideas about the equivalence of the differential as the main linear part of the increment to the increment itself and about the properties of equivalent infinitesimal quantities.
Back in 1642 he designed a calculating machine for two arithmetic operations. The principles underlying this machine later became the starting points in the design of calculating machines.
The unit of pressure, the pascal, is named after him.
Alessandro Volta, inventor of the Voltaic column, electrophorus, electrometer
Alessandro Volta was born on February 18, 1745 in the small Italian city of Como, located near Lake Como, not far from Milan. His interest in studying electrical phenomena awoke early. In 1769 he published a work on the Leyden jar, and two years later - on an electric machine. In 1774, Volta became a physics teacher at a school in Como, inventing the electrophorus, then the eudiometer and other instruments. In 1777 he became professor of physics in Pavia. In 1783 he invents an electroscope with a capacitor, and since 1792 he has been intensively working on “animal electricity”. These studies led him to the invention of the first voltaic cell.
In 1800 he built the first electric current generator - volt pole. This invention brought him worldwide fame. He was elected a member of the Paris and other academies, Napoleon made him a count and senator of the Kingdom of Italy. But after his great discovery, Volta did not do anything significant in science. In 1819, he left his professorship and lived in his hometown of Como, where he died on March 5, 1827 (on the same day as Laplace and in the same year as Fresnel).
Voltaic pole
Having begun work on “animal electricity” in 1792, Volta repeated and developed Galvani’s experiments, fully accepting his point of view. But already in one of the first letters sent from Milan on April 3, 1792, he indicates that the muscles of the frog are very sensitive to electricity, they “react amazingly to electricity,” completely elusive even for Bennett’s electroscope, the most sensitive of all (made from two strips of the finest sheet gold or silver). Here is the beginning of Volta's subsequent statement that "the dissected frog represents, so to speak, an animal electrometer, incomparably more sensitive than any other most sensitive electrometer."
Volta, as a result of a long series of experiments, came to the conclusion that the cause of muscle contraction was not “animal electricity”, but the contact of dissimilar metals. “The primary cause of this electric current,” writes Volta, “whatever it may be, is the metals themselves due to the fact that they are different. It is they who, in the proper sense of the word, are exciters and movers, while the animal organ, the nerves themselves, are only passive.” The electrification on contact irritates the nerves of the animal, sets the muscles in motion, causes a sensation of sour taste on the tip of the tongue, placed between the tin paper and the silver spoon, when the silver and tin come into contact. Thus, Volta considers the causes of “galvanism” to be physical, and physiological actions to be one of the manifestations of this physical process. If we briefly formulate Volta's thought in modern language, it boils down to the following: Galvani discovered the physiological effect of electric current.
Naturally, a controversy broke out between Galvani and Volta. To prove that he was right, Galvani tried to completely exclude physical causes. Volta, on the other hand, completely eliminated physiological objects, replacing the frog's leg with his electrometer. On February 10, 1794 he writes:
“What do you think about so-called animal electricity? As for me, I have long been convinced that all action arises initially from the contact of metals with some moist body or with water itself. Due to such contact, the electric fluid is driven into this wet body or into water from the metals themselves, from one more, from another less (most of all from zinc, least from silver). When a continuous communication is established between the corresponding conductors, this fluid undergoes a constant circulation.”
Volta devices
This is the first description of a closed circuit of electric current. If the chain is broken and a viable frog nerve is inserted into the place of the break as a connecting link, then “the muscles controlled by such nerves begin to contract as soon as the chain of conductors is closed and an electric current appears.” As we see, Volta already uses such a term as “closed circuit of electric current”. It shows that the presence of current in a closed circuit can also be detected by taste sensations if the tip of the tongue is inserted into the circuit. “And these sensations and movements are the stronger, the further the two metals used are spaced from each other in the row in which they are placed here: zinc, tin foil, ordinary tin in plates, lead, iron, brass and bronze, copper of various qualities, platinum, gold, silver, mercury, graphite.” This is the famous “Volta series” in its first draft.
Volta divided conductors into two classes. He classified metals as the first, and liquid conductors as the second. If you make a closed circuit of dissimilar metals, then there will be no current - this is a consequence of Volta’s law for contact voltages. If “a conductor of the second class is in the middle and comes into contact with two conductors of the first class made of two different metals, then as a result an electric current arises in one direction or another.”
It is quite natural that it was Volta who had the honor of creating the first generator of electric current, the so-called Voltaic column (Volta himself called it “electric organ”), which had a huge impact not only on the development of the science of electricity, but also on the entire history of human civilization. The Voltaic Column heralded the advent of a new era - the era of electricity.
Electrophor Volta
The triumph of the Voltaic pillar ensured the unconditional victory of Volta over Galvani. History was wise to determine the winner in this dispute, in which both sides were right, each from their own point of view. “Animal electricity” does exist, and electrophysiology, of which Galvani was the father, now occupies an important place in science and practice. But in Galvani's time, electrophysiological phenomena were not yet ripe for scientific analysis, and the fact that Volta turned Galvani's discovery onto a new path was very important for the young science of electricity. By excluding life - this most complex natural phenomenon - from the science of electricity, giving physiological actions only the passive role of a reagent, Volta ensured the rapid and fruitful development of this science. This is his immortal merit in the history of science and humanity.
Heinrich Rudolf Hertz, inventor of the "Hertz vibrator"
HEINRICH RUDOLF HERZ(1857-1894) was born on February 22 in Hamburg, in the family of a lawyer who later became a senator. Hertz studied well and was an unsurpassed student in intelligence. He loved all subjects, loved to write poetry and work on a lathe. Unfortunately, Hertz was hampered by poor health throughout his life.
In 1875, after graduating from high school, Hertz entered the Dresden and then the Munich Higher Technical School. Things went well as long as general subjects were studied. But as soon as specialization began, Hertz changed his mind. He does not want to be a narrow specialist, he is eager for scientific work and enters the University of Berlin. Hertz was lucky: Helmholtz turned out to be his immediate mentor. Although the famous physicist was an adherent of the theory of long-range action, as a true scientist he unconditionally recognized that the ideas of Faraday and Maxwell about short-range action and the physical field gave excellent agreement with experiment.
Once at the University of Berlin, Hertz eagerly strove to study in physics laboratories. But only those students who were engaged in solving competitive problems were allowed to work in laboratories. Helmholtz proposed to Hertz a problem from the field of electrodynamics: does an electric current have kinetic energy? Helmholtz wanted to direct Hertz's forces to the field of electrodynamics, considering it the most confusing.
Hertz sets about solving the problem, which will take 9 months. He makes the instruments himself and debugs them. When working on the first problem, the researcher traits inherent in Hertz immediately emerged: perseverance, rare diligence and the art of an experimenter. The problem was solved in 3 months. The result, as expected, was negative. (Now it is clear to us that electric current, which is the directed movement of electric charges (electrons, ions), has kinetic energy. In order for Hertz to detect this, it was necessary to increase the accuracy of his experiment thousands of times.) The result obtained coincided with the point of view Helmholtz, although erroneous, was not mistaken in the abilities of young Hertz. “I saw that I was dealing with a student of completely unusual talent,” he later noted. Hertz's work was awarded a prize.
Returning from the summer holidays in 1879, Hertz obtained permission to work on another topic:<0б индукции во вращающихся телах«, взятой в качестве докторской диссертации. Это была теоретическая работа. Он предполагал завершить ее за 2-3 месяца, защитить и получить поскорее звание доктора, хотя университет еще не был закончен. Работая с большим подъемом и воодушевлением, Герц быстро закончил исследование. Зашита прошла успешно, и ему присудили степень доктора с «отличием» - явление исключительно редкое, тем более для студента.
From 1883 to 1885, Hertz headed the department of theoretical physics in the provincial town of Kiel, where there was no physical laboratory at all. Hertz decided to deal with theoretical issues here. He corrects the system of electrodynamics equations of one of the brightest representatives of Neumann's long-range action. As a result of this work, Hertz wrote his own system of equations, from which Maxwell's equations were easily obtained. Hertz is disappointed, because he tried to prove the universality of the electrodynamic theories of representatives of long-range action, and not Maxwell’s theory. “This conclusion cannot be considered an exact proof of the Maxwellian system as the only possible one,” he draws an essentially reassuring conclusion for himself.
In 1885, Hertz accepted an invitation from the technical school in Karlsruhe, where his famous experiments on the propagation of electric force would be carried out. Back in 1879, the Berlin Academy of Sciences set the task: “To demonstrate experimentally the presence of any connection between electrodynamic forces and the dielectric polarization of dielectrics.” Hertz's preliminary calculations showed that the expected effect would be very small even under the most favorable conditions. Therefore, apparently, he abandoned this work in the fall of 1879. However, he did not stop thinking about possible ways to solve it and came to the conclusion that this required high-frequency electrical oscillations.
Hertz carefully studied everything that was known by this time about electrical oscillations, both theoretically and experimentally. Having found a pair of induction coils in the physics room of a technical school and conducting lecture demonstrations with them, Hertz discovered that with their help it was possible to obtain fast electrical oscillations with a period of 10 -8 C. As a result of the experiments, Hertz created not only a high-frequency generator (a source of high-frequency oscillations) , but the resonator is also a receiver of these vibrations.
The Hertz generator consisted of an induction coil and wires connected to it, forming a discharge gap; a resonator was made of a rectangular wire and two balls at its ends, also forming a discharge gap. As a result of his experiments, Hertz discovered that if high-frequency oscillations occur in the generator (a spark jumps in its discharge gap), then in the discharge gap of the resonator, even 3 m away from the generator , There will also be small sparks. Thus, a spark occurred in the second circuit without any direct contact with the first circuit. What is the mechanism of its transmission? Or is it electrical induction, according to Helmholtz's theory, or an electromagnetic wave, according to Maxwell's theory? In 1887, Hertz has not yet said anything about electromagnetic waves, although he has already noticed that the influence of the generator on the receiver is especially strong in the case of resonance (the oscillation frequency of the generator coincides with the natural frequency of the resonator).
After conducting numerous experiments at various relative positions of the generator and receiver, Hertz came to the conclusion about the existence of electromagnetic waves propagating at a finite speed. Will they behave like light? And Hertz is conducting a thorough test of this assumption. After studying the laws of reflection and refraction, after establishing polarization and measuring the speed of electromagnetic waves, he proved their complete analogy with light waves. All this was outlined in the work “On the Rays of Electric Force,” published in December 1888. This year is considered the year of the discovery of electromagnetic waves and the experimental confirmation of Maxwell’s theory. In 1889, speaking at a congress of German naturalists, Hertz said: “All these experiments are very simple in principle, nevertheless they entail the most important consequences. They destroy every theory that believes that electrical forces jump over space instantly. They signify a brilliant victory for Maxwell's theory. As unlikely as her view of the essence of light previously seemed, it is now so difficult not to share this view.”
Hertz's hard work did not go unpunished for his already poor health. First my eyes failed, then my ears, teeth and nose started to hurt. Soon, general blood poisoning began, from which the famous scientist Heinrich Hertz died at the age of 37.
Hertz completed the enormous work begun by Faraday. If Maxwell transformed Faraday's ideas into mathematical images, then Hertz turned these images into visible and audible electromagnetic waves, which became his eternal monument. We remember G. Hertz when we listen to the radio, watch TV, when we rejoice at the TASS report about new launches of spacecraft, with which stable communication is maintained using radio waves. And it is no coincidence that the first words transmitted by the Russian physicist A. S. Popov over the first wireless communication were: “Heinrich Hertz.”
"Very fast electrical oscillations"
Heinrich Rudolf Hertz, 1857-1894
Between 1886 and 1888, Hertz, in the corner of his physics office at the Polytechnic School of Karlsruhe (Berlin), investigated the emission and reception of electromagnetic waves. For these purposes, he invented and designed his famous emitter of electromagnetic waves, later called the “Hertz vibrator.” The vibrator consisted of two copper rods with brass balls mounted on the ends and one large zinc sphere or square plate, which played the role of a capacitor. There was a gap between the balls - a spark gap. The ends of the secondary winding of the Ruhmkorff coil, a converter of low voltage direct current to high voltage alternating current, were attached to the copper rods. With alternating current pulses, sparks jumped between the balls and electromagnetic waves were emitted into the surrounding space. By moving spheres or plates along the rods, the inductance and capacitance of the circuit, which determine the wavelength, were regulated. To capture emitted waves, Hertz came up with the simplest resonator - a wire open ring or a rectangular open frame with the same brass balls at the ends as the “transmitter” and an adjustable spark gap.
Hertz vibrator
The concept of a Hertz vibrator is introduced, a working diagram of a Hertz vibrator is given, and the transition from a closed loop to an electric dipole is considered
Using a vibrator, a resonator and reflective metal screens, Hertz proved the existence of electromagnetic waves propagating in free space, predicted by Maxwell. He proved their identity with light waves (the similarity of the phenomena of reflection, refraction, interference and polarization) and was able to measure their length.
Thanks to his experiments, Hertz came to the following conclusions: 1 - Maxwell’s waves are “synchronous” (the validity of Maxwell’s theory that the speed of propagation of radio waves is equal to the speed of light); 2 - you can transmit the energy of electric and magnetic fields wirelessly.
In 1887, upon completion of the experiments, Hertz’s first article “On very fast electrical oscillations” was published, and in 1888 an even more fundamental work “On electrodynamic waves in the air and their reflection” was published.
Hertz believed that his discoveries were no more practical than Maxwell’s: “This is absolutely useless. This is just an experiment that proves that Maestro Maxwell was right. We just have mysterious electromagnetic waves that we can’t see with our eyes, but they are there.” “So what next?” - one of the students asked him. Hertz shrugged, he was a modest man, without pretensions or ambitions: “I guess - nothing.”
But even at the theoretical level, Hertz’s achievements were immediately noted by scientists as the beginning of a new “electrical era.”
Heinrich Hertz died at the age of 37 in Bonn from blood poisoning. After Hertz's death in 1894, Sir Oliver Lodge remarked: “Hertz did what eminent English physicists could not do. Besides confirming the truth of Maxwell's theorems, he did so with disconcerting modesty."
Edward Eugene Desair Branly, inventor of the "Branly sensor"
The name of Edouard Branly is not particularly well known in the world, but in France he is considered one of the most important contributors to the invention of radiotelegraph communication.
In 1890, Edouard Branly, a professor of physics at the Catholic University of Paris, became seriously interested in the possibility of using electricity in therapy. In the mornings he went to Parisian hospitals, where he carried out medical procedures with electric and induction currents, and in the afternoon he studied the behavior of metal conductors and galvanometers when exposed to electric charges in his physics laboratory.
The device that brought Branley fame was a "glass tube loosely filled with metal filings" or "Branly sensor". When the sensor was connected to an electrical circuit containing a battery and a galvanometer, it acted as an insulator. However, if an electric spark occurred at some distance from the circuit, the sensor began to conduct current. When the tube was slightly shaken, the sensor again became an insulator. The response of the Branley sensor to a spark was observed within the laboratory premises (up to 20 m). The phenomenon was described by Branley in 1890.
By the way, a similar method of changing the resistance of sawdust, only coal, when passing an electric current, was widely used until recently (and in some homes is still used today) in telephone microphones (the so-called “carbon” microphones).
According to historians, Branly never thought about the possibility of transmitting signals. He was interested mainly in the parallels between medicine and physics and sought to offer the medical world an interpretation of nerve conduction modeled using metal filings-filled tubes.
The connection between the conductivity of the Branly sensor and electromagnetic waves was first publicly demonstrated by British physicist Oliver Lodge.
Lavoisier Antoine Laurent, inventor of the calorimeter
Antoine Laurent Lavoisier was born on August 26, 1743 in Paris in the family of a lawyer. He received his initial education at Mazarin College, and in 1864 he graduated from the Faculty of Law of the University of Paris. Already while studying at the University, Lavoisier, in addition to jurisprudence, was thoroughly engaged in the natural and exact sciences under the guidance of the best Parisian professors of that time.
In 1765, Lavoisier presented a work on the topic given by the Paris Academy of Sciences - “On the best way to illuminate the streets of a big city.” When carrying out this work, Lavoisier's extraordinary persistence in pursuing the intended goal and accuracy in research were reflected - virtues that constitute the hallmark of all his works. For example, to increase the sensitivity of his vision to subtle changes in light intensity, Lavoisier spent six weeks in a dark room. This work by Lavoisier was awarded a gold medal by the Academy.
In the period 1763-1767. Lavoisier makes a series of excursions with the famous geologist and mineralogist Guettard, helping the latter in drawing up a mineralogical map of France. Already these first works of Lavoisier opened the doors of the Paris Academy for him. On May 18, 1768, he was elected to the academy as an adjunct in chemistry, in 1778 he became a full member of the academy, and from 1785 he was its director.
In 1769, Lavoisier joined the Taxation Company, an organization of forty major financiers, in exchange for the immediate payment of a certain amount to the treasury, which received the right to collect state indirect taxes (on salt, tobacco, etc.). As a tax farmer, Lavoisier made a huge fortune, part of which he spent on scientific research; however, it was participation in the Tax Farm Company that became one of the reasons why Lavoisier was sentenced to death in 1794.
In 1775, Lavoisier became director of the Office of Gunpowder and Saltpeter. Thanks to Lavoisier's energy, the production of gunpowder in France more than doubled by 1788. Lavoisier organizes expeditions to find saltpeter deposits and conducts research on the purification and analysis of saltpeter; the methods for purifying nitrate developed by Lavoisier and Baume have survived to this day. Lavoisier managed the gunpowder business until 1791. He lived in the gunpowder Arsenal; The wonderful chemical laboratory he created at his own expense was also located here, from which almost all the chemical works that immortalized his name came out. Lavoisier's laboratory was one of the main scientific centers in Paris at that time.
In the early 1770s. Lavoisier begins systematic experimental work to study combustion processes, as a result of which he comes to the conclusion that the phlogiston theory is untenable. Having received oxygen in 1774 (following K.V. Scheele and J. Priestley) and having managed to realize the significance of this discovery, Lavoisier created the oxygen theory of combustion, which he outlined in 1777. In 1775-1777. Lavoisier proves the complex composition of air, consisting, in his opinion, of “clean air” (oxygen) and “suffocating air” (nitrogen). In 1781, together with the mathematician and chemist J.B. Meunier, he also proved the complex composition of water, establishing that it consists of oxygen and “combustible air” (hydrogen). In 1785, they synthesized water from hydrogen and oxygen.
The doctrine of oxygen as the main combustion agent was initially met with very hostility. The famous French chemist Maceur ridicules the new theory; in Berlin, where the memory of the creator of the phlogiston theory, G. Stahl, was especially revered, Lavoisier’s works were even burned. Lavoisier, however, without initially wasting time on polemics with the view, the inconsistency of which he felt, step by step persistently and patiently established the foundations of his theory. Only after carefully studying the facts and finally clarifying his point of view, Lavoisier in 1783 openly criticized the doctrine of phlogiston and showed its instability. The establishment of the composition of water was a decisive blow to the theory of phlogiston; its supporters began to go over to the side of Lavoisier’s teachings.
Based on the properties of oxygen compounds, Lavoisier was the first to give a classification of “simple bodies” known at that time in chemical practice. Lavoisier's concept of elementary bodies was purely empirical: Lavoisier considered elementary bodies to be those bodies that could not be decomposed into simpler components.
The basis for his classification of chemical substances, together with the concept of simple bodies, were the concepts of “oxide”, “acid” and “salt”. According to Lavoisier, an oxide is a compound of a metal with oxygen; acid - a compound of a non-metallic body (for example, coal, sulfur, phosphorus) with oxygen. Lavoisier considered organic acids - acetic, oxalic, tartaric, etc. - as compounds with oxygen of various “radicals”. A salt is formed by combining an acid with a base. This classification, as further research soon showed, was narrow and therefore incorrect: some acids, such as hydrocyanic acid, hydrogen sulfide, and their corresponding salts, did not fit these definitions; Lavoisier considered hydrochloric acid a compound of oxygen with an as yet unknown radical, and considered chlorine as a compound of oxygen with hydrochloric acid. Nevertheless, this was the first classification that made it possible to survey with great simplicity a whole series of bodies known at that time in chemistry. She gave Lavoisier the opportunity to predict the complex composition of such bodies as lime, barite, caustic alkalis, boric acid, etc., which before him were considered elementary bodies.
In connection with the abandonment of the phlogiston theory, the need arose to create a new chemical nomenclature, which was based on the classification given by Lavoisier. Lavoisier developed the basic principles of the new nomenclature in 1786-1787. together with C.L. Berthollet, L.B. Guiton de Morveau and A.F. Fourcroix. The new nomenclature brought greater simplicity and clarity to the chemical language, clearing it of the complex and confusing terms that were bequeathed by alchemy. Since 1790, Lavoisier also took part in the development of a rational system of measures and weights - the metric one.
The subject of Lavoisier's study was also thermal phenomena closely related to the combustion process. Together with Laplace, the future creator of Celestial Mechanics, Lavoisier gives rise to calorimetry. They create ice calorimeter, with the help of which the heat capacities of many bodies and the heat released during various chemical transformations are measured. Lavoisier and Laplace in 1780 established the basic principle of thermochemistry, which they formulated in the following form: “Any thermal changes that any material system experiences, changing its state, occur in the reverse order, when the system returns to its original state.”
In 1789, Lavoisier published the textbook “Elementary Course of Chemistry,” based entirely on the oxygen theory of combustion and new nomenclature, which became the first textbook of new chemistry. Since the French Revolution began in the same year, the revolution accomplished in chemistry by the works of Lavoisier is usually called the “chemical revolution.”
The creator of the chemical revolution, Lavoisier became, however, a victim of the social revolution. At the end of November 1793, the former participants in the tax farming were arrested and tried by a revolutionary tribunal. Neither a petition from the Advisory Bureau of Arts and Crafts, nor well-known services to France, nor scientific fame saved Lavoisier from death. “The Republic does not need scientists,” said the president of the Coffinal tribunal in response to the bureau’s petition. Lavoisier was accused of participating “in a conspiracy with the enemies of France against the French people, aimed at stealing from the nation huge sums necessary for the war against despots,” and was sentenced to death. “The executioner had only a moment to cut off this head,” said the famous mathematician Lagrange regarding the execution of Lavoisier, “but a century will not be enough to give another like it...” In 1796, Lavoisier was posthumously rehabilitated.
Since 1771, Lavoisier was married to the daughter of his fellow farmer, Benefit. In his wife he found an active assistant in his scientific work. She kept his laboratory journals, translated scientific articles for him from English, and drew and engraved drawings for his textbook. After Lavoisier's death, his wife remarried in 1805 to the famous physicist Rumfoord. She died in 1836 at the age of 79.
Pierre Simon Laplace, inventor of the calorimeter, barometric formula
French astronomer, mathematician and physicist Pierre Simon de Laplace was born in Beaumont-en-Auge, Normandy. He studied at the Benedictine school, from which he emerged, however, as a convinced atheist. In 1766, Laplace arrived in Paris, where J. d'Alembert five years later helped him get a position as a professor at the Military School. He actively participated in the reorganization of the higher education system in France, in the creation of the Normal and Polytechnic schools. In 1790, Laplace was appointed chairman of the Chamber of Weights and Measures and led the introduction of a new metric system of measures. Since 1795, as part of the leadership of the Bureau of Longitudes. Member of the Paris Academy of Sciences (1785, adjunct from 1773), member of the French Academy (1816).
Laplace's scientific heritage relates to the field of celestial mechanics, mathematics and mathematical physics; Laplace's work on differential equations is fundamental, in particular on the integration of partial differential equations using the “cascade” method. The spherical functions introduced by Laplace have various applications. In algebra, Laplace has an important theorem on the representation of determinants by the sum of products of additional minors. To develop the mathematical theory of probability that he created, Laplace introduced the so-called generating functions and widely used the transformation that bears his name (the Laplace transform). Probability theory was the basis for the study of all kinds of statistical patterns, especially in the field of natural science. Before him, the first steps in this area were taken by B. Pascal, P. Fermat, J. Bernoulli and others. Laplace brought their conclusions into a system, improved the methods of proof, making them less cumbersome; proved the theorem that bears his name (Laplace's theorem), developed the theory of errors and the method of least squares, which make it possible to find the most probable values of measured quantities and the degree of reliability of these calculations. Laplace's classic work, The Analytical Theory of Probability, was published three times during his lifetime - in 1812, 1814 and 1820; As an introduction to the latest editions, the work “An Experience in the Philosophy of the Theory of Probability” (1814) was placed, in which the basic provisions and significance of the theory of probability are explained in a popular form.
Together with A. Lavoisier in 1779-1784. Laplace studied physics, in particular the question of the latent heat of fusion of bodies and work with the created by them ice calorimeter. They were the first to use a telescope to measure the linear expansion of bodies; studied the combustion of hydrogen in oxygen. Laplace actively opposed the erroneous hypothesis of phlogiston. Later he returned to physics and mathematics. He published a number of works on the theory of capillarity and established the law that bears his name (Laplace's law). In 1809, Laplace took up questions of acoustics; derived a formula for the speed of sound propagation in air. belongs to Laplace barometric formula to calculate changes in air density with height above the ground, taking into account the influence of air humidity and changes in the acceleration of gravity. He was also involved in geodesy.
Laplace developed the methods of celestial mechanics and completed almost everything that his predecessors failed to explain the motion of bodies in the Solar System on the basis of Newton’s law of universal gravitation; he managed to prove that the law of universal gravitation completely explains the movement of these planets if we imagine their mutual perturbations in the form of series. He also proved that these disturbances are periodic. In 1780, Laplace proposed a new method for calculating the orbits of celestial bodies. Laplace's research proved the stability of the solar system for a very long time. Next, Laplace came to the conclusion that Saturn’s ring cannot be continuous, because in this case it would be unstable, and predicted the discovery of a strong compression of Saturn at the poles. In 1789, Laplace considered the theory of the motion of Jupiter's satellites under the influence of mutual disturbances and attraction to the Sun. He obtained complete agreement between theory and observations and established a number of laws for these movements. One of Laplace's main achievements was the discovery of the cause of acceleration in the motion of the Moon. In 1787, he showed that the average speed of the Moon depends on the eccentricity of the Earth's orbit, and the latter changes under the influence of the gravity of the planets. Laplace proved that this disturbance is not secular, but long-period, and that subsequently the Moon will begin to move slowly. From the inequalities in the motion of the Moon, Laplace determined the amount of compression of the Earth at the poles. He also developed the dynamic theory of tides. Celestial mechanics owes much to the works of Laplace, which he summarized in his classic work “Treatise on Celestial Mechanics” (vols. 1-5, 1798-1825).
Laplace's cosmogonic hypothesis had enormous philosophical significance. It is outlined by him in the appendix to his book “Exposition of the World System” (vol. 1-2, 1796).
In his philosophical views, Laplace was aligned with the French materialists; Laplace's answer to Napoleon I is known that in his theory about the origin of the solar system he did not need the hypothesis of the existence of God. The limitations of Laplace's mechanistic materialism manifested themselves in an attempt to explain the entire world, including physiological, mental and social phenomena, from the point of view of mechanistic determinism. Laplace considered his understanding of determinism as a methodological principle for the construction of any science. Laplace saw an example of the final form of scientific knowledge in celestial mechanics. Laplace determinism became a common name for the mechanistic methodology of classical physics. Laplace's materialistic worldview, clearly reflected in his scientific works, contrasts with his political instability. With every political revolution, Laplace went over to the winning side: at first he was a republican, after Napoleon came to power - the Minister of the Interior; then he was appointed a member and vice-chairman of the Senate, under Napoleon he received the title of Count of the Empire, and in 1814 he cast his vote for the deposition of Napoleon; After the Bourbon restoration, he received a peerage and the title of marquis.
Oliver Joseph Lodge, inventor of the coherer
Among Lodge's major contributions in the context of radio is his improvement of the Branly radio wave sensor.
Lodge's coherer, first demonstrated to an audience at the Royal Institution in 1894, allowed Morse code signals transmitted by radio waves to be received and recorded by a recording apparatus. This allowed the invention to soon become a standard device for wireless telegraph devices. (The sensor would not fall out of use until ten years later, when magnetic, electrolytic and crystalline sensors would be developed).
No less important is Lodge's other work in the field of electromagnetic waves. In 1894, Lodge, in the pages of the London Electrician, discussing the significance of Hertz's discoveries, described his experiments with electromagnetic waves. He commented on the phenomenon of resonance or tuning he discovered:
... some circuits are “vibrating” in nature... They are able to maintain the vibrations that arise in them for a long period, while in other circuits the vibrations quickly die out. A damped receiver will respond to waves of any frequency, as opposed to a constant frequency receiver, which responds only to waves at its own frequency.
Lodge found that the Hertz vibrator "radiated very powerfully" but "because of the radiation of energy (into space), its oscillations are rapidly damped, so that in order to transmit a spark it must be tuned in accordance with the receiver."
On August 16, 1898, Lodge received Patent No. 609154, which proposed "the use of a tunable telecoil or antenna circuit in wireless transmitters or receivers, or both." This "syntonic" patent was of great importance in the history of radio because it outlined the principles of tuning to the desired station. On March 19, 1912, this patent was acquired by the Marconi company.
Subsequently, Marconi said this about Lodge:
He (Lodge) is one of our greatest physicists and thinkers, but his work in the field of radio is especially significant. From the earliest days, after the experimental confirmation of Maxwell's theory regarding the existence of electromagnetic radiation and its propagation through space, very few people had a clear understanding regarding the solution to this one of the most hidden mysteries of nature. Sir Oliver Lodge had this understanding to a much greater degree than any other of his contemporaries.
Why didn't Lodge invent the radio? He himself explained this fact this way:
I was too busy with work to take on the development of the telegraph or any other branch of technology. I did not have sufficient understanding to sense how extraordinarily important this would be for the navy, commerce, civil and military communications.
For his contribution to the development of science, Lodge was knighted by King Edward VII in 1902.
The further fate of Sir Oliver is interesting and mysterious.
After 1910, he became interested in spiritualism and became an ardent supporter of the idea of communicating with the dead. He was interested in the connection between science and religion, telepathy, and manifestations of the mysterious and unknown. In his opinion, the easiest way to communicate with Mars would be to move giant geometric shapes across the Sahara Desert. At the age of eighty, Lodge announced that he would attempt to contact the world of the living after his death. He handed over a sealed document for safekeeping to the English Society for Psychical Research, which, according to him, contained the text of the message that he would convey from the other world.
Luigi Galvani, inventor of the galvanometer
Luigi Galvani was born in Bologna on September 9, 1737. He studied first theology, and then medicine, physiology and anatomy. In 1762 he was already a teacher of medicine at the University of Bologna.
In 1791, Galvani's famous discovery was described in his Treatise on the Forces of Electricity in Muscular Movement. The phenomena themselves discovered by Galvani were called for a long time in textbooks and scientific articles "galvanism". This term is still preserved in the names of some devices and processes. Galvani himself describes his discovery as follows:
“I cut and dissected the frog... and, having something completely different in mind, placed it on the table on which there was an electric machine..., completely separated from the conductor of the latter and at a fairly large distance from him. When one of my assistants, with the tip of a scalpel, accidentally very lightly touched the internal femoral nerves of this frog, immediately all the muscles of the limbs began to contract so much that they seemed to have fallen into severe tonic convulsions. Another of them, who helped us in experiments on electricity, noticed how he it seemed that this was successful when a spark was drawn from the conductor of the machine... Surprised by the new phenomenon, he immediately drew my attention to it, although I was planning something completely different and was absorbed in my thoughts. Then I was fired with incredible zeal and a passionate desire to explore this phenomenon and bring to light what was hidden in it.”
This description, classic in its accuracy, has been repeatedly reproduced in historical works and has given rise to numerous commentaries. Galvani honestly writes that the phenomenon was first noticed not by him, but by two of his assistants. It is believed that the “other present” who indicated that muscle contraction occurs when a spark jumps in the machine was his wife Lucia. Galvani was busy with his thoughts, and at this time someone began to rotate the handle of the machine, someone touched the drug “lightly” with a scalpel, someone noticed that muscle contraction occurs when a spark jumps. Thus, in a chain of accidents (all the characters hardly conspired with each other), a great discovery was born. Galvani was distracted from his thoughts, “he himself began to touch with the tip of a scalpel first one or the other femoral nerve, while one of those present extracted a spark, the phenomenon occurred in exactly the same way.”
As we can see, the phenomenon was very complex; three components came into play: an electric machine, a scalpel, and a frog’s leg preparation. What is essential? What happens if one of the components is missing? What is the role of the spark, the scalpel, the frog? Galvani tried to get an answer to all these questions. He conducted numerous experiments, including outdoors during a thunderstorm. “And so, sometimes noticing that the dissected frogs, which were suspended on the iron grating that surrounded the balcony of our house, with the help of copper hooks stuck into the spinal cord, fell into the usual contractions not only in a thunderstorm, but sometimes also in a calm and clear sky , I decided that these contractions were caused by changes occurring during the day in atmospheric electricity." Galvani goes on to describe how he waited in vain for these cuts. “Finally tired of waiting in vain, I began to press the copper hooks stuck into the spinal cord against the iron lattice,” and here I discovered the desired contractions, which occurred without any changes “in the state of the atmosphere and electricity.”
Galvani transferred the experiment to the room, placed the frog on an iron plate, against which he began to press a hook drawn through the spinal cord, muscle contractions immediately appeared. This was the decisive discovery.
Galvani realized that something new had opened up before him and decided to carefully investigate the phenomenon. He felt that in such cases “it is easy to make a mistake with research and consider what we want to see and find to be seen and found,” in this case the influence of atmospheric electricity. He transferred the drug “to a closed room, placed it on an iron plate and began to press it against it.” a hook passed through the spinal cord.” At the same time, “the same contractions, the same movements appeared.” So, there is no electric machine, no atmospheric discharges, and the effect is observed as before. “Of course,” writes Galvani, “such a result caused us considerable surprise and began to arouse in us some suspicion about the electricity inherent in the animal itself.” To test the validity of such a “suspicion,” Galvani performed a series of experiments, including a spectacular experiment when a suspended paw, touching a silver plate, contracts, is pressed up, then falls, contracts again, etc. “So this paw, “- writes Galvani, “to the considerable admiration of those who watch it, it seems to begin to compete with some kind of electric pendulum.”
Galvani's suspicion turned into confidence: the frog's leg became for him a carrier of “animal electricity”, like a charged Leyden jar. “After these discoveries and observations, it seemed to me possible to conclude without any delay that this dual and opposing electricity is found in the animal preparation itself.” He showed that positive electricity is in the nerve, negative electricity is in the muscle.
It is quite natural that the physiologist Galvani came to the conclusion about the existence of “animal electricity”. The whole experimental situation pushed towards this conclusion. But the physicist, who first believed in the existence of “animal electricity,” soon came to the opposite conclusion about the physical cause of the phenomenon. This physicist was Galvani's famous compatriot Alessandro Volta.
John Ambrose Fleming, inventor of the wave meter
English engineer John Fleming made significant contributions to the development of electronics, photometry, electrical measurements and radiotelegraph communications. Most famous is his invention of a radio detector (rectifier) with two electrodes, which he called the thermionic tube, also known as a vacuum diode, kenotron, electron tube and tube or Fleming diode. This device, patented in 1904, was the first electronic radio wave detector to convert alternating current radio signals to direct current. Fleming's discovery was the first step in the era of vacuum tube electronics. An era that lasted almost until the end of the 20th century.
Fleming studied at University College in London and in Cambridge with the great Maxwell, and for many years worked as a consultant for the London companies of Edison and Marconi.
He was a very popular teacher at University College and the first to be awarded the title of Professor of Electrical Engineering. He was the author of more than a hundred scientific articles and books, including the popular Principles of Electrical Wave Telegraphy (1906) and The Propagation of Electric Currents in Telephone and Telegraph Wires (1911), which were the leading books on the subject for many years. In 1881, as electricity began to attract widespread attention, Fleming joined the Edison Company in London as an electrical engineer, which he held for almost ten years.
It was natural that Fleming's work on electricity and telephony should sooner or later lead him into the nascent radio engineering. For more than twenty-five years he served as a scientific advisor to the Marconi company and even took part in the creation of the first transatlantic station in Poldu.
For a long time, controversy continued over the wavelength at which the first transatlantic transmission was carried out. In 1935, in his memoirs, Fleming commented on this fact:
“In 1901, the wavelength of electromagnetic radiation was not measured, because by that time I had not yet invented wave meter(invented in October 1904). The height of the antenna suspension in the first version was 200 feet (61 m). We connected a transformer coil or “jiggeroo” (damped oscillation transformer) in series with the antenna. I estimate that the original wavelength must have been at least 3,000 feet (915 m), but later it was much higher.
At that time I knew that diffraction, the bending of waves around the earth, would increase with wavelength and after the initial success I constantly urged Marconi to increase the wavelength, which was done when commercial transmissions began. I remember that I developed special wave meters to measure waves of about 20,000 feet (6096 m)."
Pold's triumph belonged to Marconi, and Fleming's fame was brought to him by the “small incandescent electric lamp” - the Fleming diode. He himself described this invention as follows:
“In 1882, as an electrical adviser to the Edison Electric Light Company of London, I solved numerous problems with incandescent lamps and began to study the physical phenomena occurring in them with all the technical means at my disposal. Like many others, I noticed that the filaments broke easily with small impacts and that after the lamps burned out, their glass bulbs changed color. This change in glass was so common that it was taken for granted by everyone. It seemed trivial to pay attention to this. But in science, every little detail must be taken into account. Little things today and tomorrow can make a huge difference.
Wondering why the bulb of an incandescent lamp turned dark, I began to research this fact and discovered that many burned out lamps had a strip of glass that did not change color. It looked like someone had taken a sooty flask and wiped away the residue, leaving a narrow strip clean. I determined that the lamps with these strange, sharply defined clear areas were elsewhere coated with deposited carbon or metal. And the clean strip was certainly U-shaped, repeating the shape of the carbon filament, and exactly on the side of the flask opposite the burnt filament.
It became obvious to me that the unbroken part of the filament acted as a screen, leaving that very characteristic strip of pure glass, and that charges from the heated filament bombarded the walls of the lamp with molecules of carbon or evaporated metal. My experiments in late 1882 and early 1883 proved that I was right."
Edison also noticed this phenomenon, by the way called the “Edison effect,” but could not explain its nature.
In October 1884, William Preece was engaged in research into the “Edison effect”. He decided that this was due to the emission of carbon molecules from the filament in straight directions, thus confirming my original discovery. But Preece, like Edison, also did not search for the truth. He did not explain the phenomenon and did not seek to apply it. The “Edison Effect” remained the mystery of the incandescent lamp.
In 1888, Fleming received several special carbon incandescent lamps made in England by Edison and Joseph Swan and continued his experiments. He applied a negative voltage to a carbon filament and noticed that the bombardment of charged particles stopped.
When the position of the metal plate changed, the intensity of the bombardment changed. When, instead of a plate, a metal cylinder was placed in the flask, located around the negative contact of the thread without contact with it, the galvanometer recorded the greatest current.
It became apparent to Fleming that the metal cylinder was "capturing" the charged particles that the thread emitted. Having thoroughly studied the properties of the effect, he discovered that the combination of a filament and a plate, called an anode, could be used as a rectifier of alternating currents not only of industrial, but also of high frequencies used in radio.
Fleming's work at Marconi's company allowed him to become thoroughly familiar with the capricious coherer used as a wave sensor. In search of a better sensor, he tried to develop chemical detectors, but at some time the thought came to him: “Why not try a lamp?”
Fleming described his experiment this way:
“It was approximately 5 o’clock in the evening when the apparatus was completed. Of course, I really wanted to test it in action. In the laboratory, we installed these two circuits at some distance from each other, and I started oscillations in the main circuit. To my delight I saw that the arrow galvanometer showed a stable constant current. I realized that we had obtained in this specific form of electric lamp a solution to the problem of rectifying high-frequency currents. The “missing part” in the radio was found and it was an electric lamp!
First, he assembled an oscillating circuit, with two Leyden jars in a wooden case and an induction coil. Then another circuit that included a vacuum tube and a galvanometer. Both circuits were tuned to the same frequency.
I immediately realized that the metal plate had to be replaced by a metal cylinder covering the entire filament to "collect" all the emitted electrons.
I had a variety of carbon incandescent lamps with metal cylinders, and I began to use them as high-frequency rectifiers for radiotelegraph communications.
I called this device an oscillating lamp. A use was immediately found for it. Galvanometer replaced with a regular phone. A replacement that could have been made at that time, taking into account the development of technology, when spark communication systems were widely used. In this form, my lamp was widely used by the Marconi company as a wave sensor. On November 16, 1904 I applied for a patent in Great Britain.
Fleming received many honors and awards for his invention of the vacuum diode. In March 1929 he was knighted for his "invaluable contribution to science and industry"
Metric system, decimal system of measures, a set of units of physical quantities, which is based on the unit of length - meter. Initially, the Metric system of measures, in addition to the meter, included the following units: area - square meter, volume - cubic meter and mass - kilogram (mass of 1 dm 3 of water at 4 ° C), as well as liter(for capacity), ar(for land area) and ton(1000 kg). An important distinctive feature of the Metric system of measures was the method of formation multiples of units And submultiple units, which are in decimal ratios; To form the names of derived units, prefixes were adopted: kilo, hecto, soundboard, deci, centi And Milli.
The metric system of measures was developed in France during the French Revolution. At the suggestion of a commission of major French scientists (J. Borda, J. Condorcet, P. Laplace, G. Monge, etc.), the unit of length - the meter - was adopted as a ten-millionth part of 1/4 of the length of the Parisian geographical meridian. This decision was determined by the desire to base the Metric system of measures on an easily reproducible “natural” unit of length associated with some practically unchanging object of nature. The decree introducing the metric system of measures in France was adopted on April 7, 1795. In 1799, a platinum prototype of the meter was manufactured and approved. The dimensions, names and definitions of other units of the Metric system of measures were chosen so that it was not national in nature and could be adopted by all countries. The metric system of measures acquired a truly international character in 1875, when 17 countries, including Russia, signed metric convention to ensure international unity and improvement of the metric system. The metric system of measures was approved for use in Russia (optional) by the law of June 4, 1899, the draft of which was developed by D. I. Mendeleev, and introduced as mandatory by the decree of the Council of People's Commissars of the RSFSR of September 14, 1918, and for the USSR - by decree Council of People's Commissars of the USSR dated July 21, 1925.
Based on the Metric system of measures, a whole series of particular measures arose, covering only certain sections of physics or branches of technology, systems of units and individual non-system units. The development of science and technology, as well as international relations, led to the creation, based on the Metric system of measures, of a unified system of units covering all areas of measurement - International System of Units(SI), which has already been accepted as mandatory or preferred by many countries.