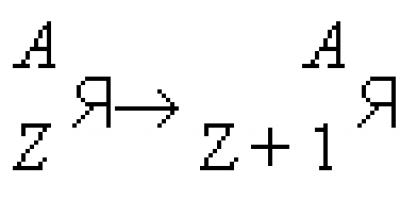Beta decay β-decay, radioactive decay of an atomic nucleus, accompanied by the emission of an electron or positron from the nucleus. This process is caused by the spontaneous transformation of one of the nucleons of the nucleus into a nucleon of a different kind, namely: the transformation of either a neutron (n) into a proton (p), or a proton into a neutron. In the first case, an electron (e -) flies out of the nucleus - the so-called β - decay occurs. In the second case, a positron (e +) flies out of the nucleus - β + decay occurs. Departing under B.-r. electrons and positrons are collectively called beta particles. The mutual transformations of nucleons are accompanied by the appearance of another particle - the neutrino ( ν
) in the case of β+ decay or antineutrino A, equal to the total number of nucleons in the nucleus, does not change, and the nuclear product is an isobar of the original nucleus, standing next to it to the right in the periodic system of elements. On the contrary, during β + -decay, the number of protons decreases by one, and the number of neutrons increases by one, and an isobar is formed, which is adjacent to the left of the original nucleus. Symbolically, both processes of B.-r. are written in the following form: where -Z neutrons. The simplest example of β - decay is the transformation of a free neutron into a proton with the emission of an electron and an antineutrino (neutron half-life ≈ 13 min): A more complex example (β - decay - the decay of a heavy isotope of hydrogen - tritium, consisting of two neutrons (n) and one proton (p): Obviously, this process comes down to the β - decay of a bound (nuclear) neutron. In this case, the β-radioactive tritium nucleus turns into the nucleus of the next element in the periodic table - the nucleus of the light isotope of helium 3 2 He. An example of β + decay is the decay of the carbon isotope 11 C according to the following scheme: The transformation of a proton into a neutron inside a nucleus can also occur as a result of the proton capturing one of the electrons from the electron shell of the atom. Most often, electron capture occurs B.-r. observed in both naturally radioactive and artificially radioactive isotopes. In order for a nucleus to be unstable with respect to one of the types of β-transformation (that is, it could experience a transformation), the sum of the masses of the particles on the left side of the reaction equation must be greater than the sum of the masses of the transformation products. Therefore, with B.-r. energy is released. Energy B.-r. Eβ can be calculated from this mass difference using the relation E = mc2, Where With - speed of light in vacuum. In the case of β decay Where M - masses of neutral atoms. In the case of β+ decay, a neutral atom loses one of the electrons in its shell, the energy of the b.-r. is equal to: Where me - electron mass. Energy B.-r. distributed between three particles: electron (or positron), antineutrino (or neutrino) and nucleus; each of the light particles can carry away almost any energy from 0 to E β i.e. their energy spectra are continuous. Only during K-capture does a neutrino always carry away the same energy. So, with β - decay, the mass of the initial atom exceeds the mass of the final atom, and with β + decay this excess is at least two electron masses. Study of B.-r. Nuclei have repeatedly presented scientists with unexpected mysteries. After the discovery of radioactivity, the phenomenon of B.-r. has long been considered as an argument in favor of the presence of electrons in atomic nuclei; this assumption turned out to be in obvious contradiction with quantum mechanics (see Atomic nucleus). Then, the inconstancy of the energy of electrons emitted during B.-R. even gave rise to some physicists’ disbelief in the law of conservation of energy, because It was known that nuclei that are in states with a very definite energy participate in this transformation. The maximum energy of electrons escaping from the nucleus is exactly equal to the difference between the energies of the initial and final nuclei. But in this case, it was not clear where the energy disappears if the emitted electrons carry less energy. The assumption of the German scientist W. Pauli about the existence of a new particle - the neutrino - saved not only the law of conservation of energy, but also another important law of physics - the law of conservation of angular momentum. Since the Spins (i.e., the intrinsic moments) of the neutron and proton are equal to 1/2, then to preserve the spin on the right side of the B.-r. equations. There can only be an odd number of particles with spin 1/2. In particular, during the β - decay of a free neutron n → p + e - + ν, only the appearance of an antineutrino eliminates the violation of the law of conservation of angular momentum. B.-r. occurs in elements of all parts of the periodic table. The tendency towards β-transformation arises due to the presence of an excess of neutrons or protons in a number of isotopes compared to the amount that corresponds to maximum stability. Thus, the tendency to β + -decay or K-capture is characteristic of neutron-deficient isotopes, and the tendency to β - -decay is characteristic of neutron-rich isotopes. About 1500 β-radioactive isotopes of all elements of the periodic table are known, except for the heaviest ones (Z ≥ 102). Energy B.-r. currently known isotopes range from half-lives are in a wide range from 1.3 10 -2 sec(12 N) to Beta decay 2 10 13 years (natural radioactive isotope 180 W). Subsequent study of B.-r. has repeatedly led physicists to the collapse of old ideas. It was found that B.-r. governed by forces of a completely new nature. Despite the long period that has passed since the discovery of B.-r., the nature of the interaction that determines B.-r. has not been fully studied. This interaction was called “weak” because it is 10 12 times weaker than nuclear and 10 9 times weaker than electromagnetic (it exceeds only the gravitational interaction; see Weak interactions). Weak interaction is inherent in all elementary particles (See Elementary particles) (except for the photon). Almost half a century passed before physicists discovered that in B.-r. the symmetry between “right” and “left” may be broken. This nonconservation of spatial parity has been attributed to the properties of weak interactions. Study of B.-r. had another important side. The lifetime of the nucleus relative to the B.-r. and the shape of the spectrum of β-particles depend on the states in which the original nucleon and the product nucleon are located inside the nucleus. Therefore, the study of magnetic resonance, in addition to information about the nature and properties of weak interactions, has significantly expanded the understanding of the structure of atomic nuclei. Probability of B.-r. depends significantly on how close the states of the nucleons in the initial and final nuclei are to each other. If the state of the nucleon does not change (the nucleon seems to remain in the same place), then the probability is maximum and the corresponding transition of the initial state to the final state is called allowed. Such transitions are characteristic of B.-r. light nuclei. Light nuclei contain almost the same number of neutrons and protons. Heavier nuclei have more neutrons than protons. The states of nucleons of different types are significantly different from each other. This makes it difficult for B.-r.; transitions appear in which B.-r. occurs with low probability. The transition is also complicated by the need to change the spin of the nucleus. Such transitions are called forbidden. The nature of the transition also affects the shape of the energy spectrum of β-particles. An experimental study of the energy distribution of electrons emitted by β-radioactive nuclei (beta spectrum) is carried out using a Beta spectrometer. Examples of β spectra are shown in rice. 1
And rice. 2
. Lit.: Alpha, beta and gamma spectroscopy, ed. K. Siegbana, trans. from English, V. 4, M., 1969, ch. 22-24; Experimental Nuclear Physics, ed. E. Segre, trans. from English, vol. 3, M., 1961. E. M. Leikin. Neutron beta spectrum. The abscissa axis shows kinetic. electron energy E in kev, on the ordinate - the number of electrons N (E) in relative units (vertical bars indicate the limits of measurement errors for electrons with a given energy). Great Soviet Encyclopedia. - M.: Soviet Encyclopedia.
1969-1978
.
![]()
![]()



![]()
![]()
![]()
![]()


See what “Beta decay” is in other dictionaries:
Beta decay, radioactive transformations of atomic nuclei; in the process, nuclei emit electrons and antineutrinos (beta decay) or positrons and neutrinos (beta+ decay). Departing during B. r. electrons and positrons are collectively called. beta particles. At… … Big Encyclopedic Polytechnic Dictionary
Modern encyclopedia
Beta decay- (b decay), a type of radioactivity in which a decaying nucleus emits electrons or positrons. In electron beta decay (b), a neutron (intranuclear or free) turns into a proton with the emission of an electron and an antineutrino (see ... ... Illustrated Encyclopedic Dictionary
Beta decay- (β decay) radioactive transformations of atomic nuclei, during which the nuclei emit electrons and antineutrinos (β decay) or positrons and neutrinos (β+ decay). Departing during B. r. electrons and positrons are collectively called beta particles (β particles)... Russian encyclopedia of labor protection
- (b decay). spontaneous (spontaneous) transformations of a neutron n into a proton p and a proton into a neutron inside the at. nuclei (as well as the transformation of a free neutron into a proton), accompanied by the emission of electron e or positron e+ and electron antineutrinos... ... Physical encyclopedia
Spontaneous transformations of a neutron into a proton and a proton into a neutron inside an atomic nucleus, as well as the transformation of a free neutron into a proton, accompanied by the emission of an electron or positron and a neutrino or antineutrino. double beta decay... ... Nuclear energy terms
- (see beta) radioactive transformation of an atomic nucleus, in which an electron and an antineutrino or a positron and a neutrino are emitted; During beta decay, the electric charge of the atomic nucleus changes by one, but the mass number does not change. New dictionary... ... Dictionary of foreign words of the Russian language
beta decay- beta rays, beta decay, beta particles. The first part is pronounced [beta]... Dictionary of difficulties of pronunciation and stress in modern Russian language
Noun, number of synonyms: 1 decay (28) ASIS Dictionary of Synonyms. V.N. Trishin. 2013… Synonym dictionary
Beta decay, beta decay... Spelling dictionary-reference book
BETA DECAY- (ß decay) radioactive transformation of an atomic nucleus (weak interaction), in which an electron and an antineutrino or a positron and a neutrino are emitted; with B. r. the electric charge of the atomic nucleus changes by one, the mass (see) does not change... Big Polytechnic Encyclopedia
The nuclei of most atoms are fairly stable formations.
However, the nuclei of atoms of radioactive substances during the process of radioactive decay spontaneously transform into the nuclei of atoms of other substances. So in 1903 Rutherford discovered that radium placed in a vessel after some time turned intoradon. And additional helium appeared in the vessel.Alpha decay

During alpha decay an alpha particle is emitted (nucleus
helium atom). From a substance with the number of protons Z and neutrons N in the atomic nucleus, it turns into a substance with the number of protons Z-2 and the number of neutrons N-2, atomic mass A-4. That is, the resulting element is shifted two cells back in the periodic table.
Alpha decay is intranuclear process. As part of a heavy nucleus, due to a complex combination of nuclear and electrostatic forces, an independent α-particle is formed, which is pushed out by Coulomb forces much more actively than other nucleons. Under certain conditions, it can overcome the forces of nuclear interaction and fly out of the nucleus.
Beta decay
During beta decay an electron (β particle) is emitted. As a result of the decay of one neutron into a proton, electron and antineutrino, the composition of the nucleus increases by one proton, and the electron and antineutrino are emitted outward. Respectively, the resulting element is shifted one cell forward in the periodic table.
the resulting element is shifted one cell forward in the periodic table.
Example of β decay:

Gamma decay
 Gamma decay is the emission of gamma quanta by nuclei in an excited state, in which they have greater energy compared to the unexcited state. Nuclei can come to an excited state during nuclear reactions or during radioactive
Gamma decay is the emission of gamma quanta by nuclei in an excited state, in which they have greater energy compared to the unexcited state. Nuclei can come to an excited state during nuclear reactions or during radioactive

There are decays with the emission of a neutron, proton, cluster radioactivity and some other, very rare types of decays. But the predominant types of radioactivity are alpha, beta and gamma decay.
Decay table
Type of radioactivity | Change in nuclear charge Z | Mass number change A | Nature of the process |
The emission of an alpha particle - a system of two protons and two neutrons connected together |
|||
Mutual transformations in the nucleus of a neutron () and a proton () |
|||
β–decay |
|
||
β+-decay |
|
||
Electronic capture (e – - or K-capture) |
And – electron neutrino and antineutrino |
||
Spontaneous fission | Z – (1/2)A | A– (1/2)A | The fission of a nucleus usually into two fragments having approximately equal masses and charges |
E. Rutherford discovered two components of this radiation: a less penetrating one, called α- radiation, and more penetrating, called - radiation. The third component of uranium radiation, the most penetrating of all, was discovered later, in 1900, by Paul Willard and named γ-radiation by analogy with the Rutherford series. Rutherford and his collaborators showed that radioactivity is associated with decay

ohm of atoms (much later it became clear that we are talking about the disintegration of atomic nuclei), accompanied by the release of a certain type of radiation from them. This conclusion dealt a crushing blow to the concept of the indivisibility of atoms that had dominated in physics and chemistry.
In subsequent studies by Rutherford, it was shown that α-radiation is a flux α particles, which are nothing more than nuclei helium isotope 4 He, A
 β radiation comprises electrons And γ radiation is a stream of high-frequency electromagnetic quanta, emitted by atomic nuclei during the transition from excited to lower states.
β radiation comprises electrons And γ radiation is a stream of high-frequency electromagnetic quanta, emitted by atomic nuclei during the transition from excited to lower states. β-decay of nuclei. The theory of this phenomenon was created only in 1933 by Enrico Fermi, who used Wolfgang Pauli's hypothesis about the birth in beta decay of a neutral particle with a rest mass close to zero and called a neutrino. Fermi discovered that β-decay is due to a new type of particle interaction in nature - “weak” interaction and is associated with the processes of transformation in the parent nucleus of a neutron into a proton with the emission of an electron e - and antineutrino (β - decay), a proton into a neutron with the emission of a positron e + and neutrino ν (β + -decay), as well as with the capture of an atomic electron by a proton and the emission of neutrinos ν (electron capture).
The fourth type of radioactivity, discovered in Russia in 1940
 young physicists G.N. Flerov and K.A. Pietrzak is associated with spontaneous nuclear fission, during which some fairly heavy nuclei decay into two fragments with approximately equal masses.
young physicists G.N. Flerov and K.A. Pietrzak is associated with spontaneous nuclear fission, during which some fairly heavy nuclei decay into two fragments with approximately equal masses. But fission did not exhaust all types of radioactive transformations of atomic nuclei. Since the 50s, physicists have methodically approached the discovery of proton radioactivity in nuclei. In order for a nucleus in the ground state to spontaneously emit a proton, it is necessary that the energy of separation of the proton from the nucleus be positive. But such nuclei do not exist under terrestrial conditions, and they had to be created artificially. Russian physicists in Dubna were very close to obtaining such nuclei, but proton radioactivity was discovered in 1982 by German physicists in Darmstadt, who used the world's most powerful accelerator of multiply charged ions.
 Finally, in 1984, independent groups of scientists in England and Russia discovered the cluster radioactivity of some heavy nuclei that spontaneously emit clusters - atomic nuclei with atomic weights from 14 to 34.
Finally, in 1984, independent groups of scientists in England and Russia discovered the cluster radioactivity of some heavy nuclei that spontaneously emit clusters - atomic nuclei with atomic weights from 14 to 34.
1win is one of the popular bookmakers that offers a large selection of online sports bets. On the bookmaker's official website you can find about 20 sections of various sports.
Go to mirror
- What is a 1win mirror
Currently, players place bets using 1win mirrors. A mirror is a kind of duplicate of the main site, which has the same interface and functions except for the domain name.
The domain name is usually chosen to be similar to the address of the main site. The mirror allows the bookmaker to reduce the load on its main server by distributing players, which helps ensure a stable and uninterrupted gaming experience.
In addition, if the main 1win site is blocked by the provider or regulatory authorities, clients can turn to the mirror site and calmly continue making profitable bets. There are times when both the main site and mirrors stop working, but the bookmaker quickly solves this problem by creating 1-3 more new pages. Thus, a mirror is a website completely similar to the main one, which is created to solve several problems at once.
- Why was the 1win mirror blocked?
According to the new Federal Law of the Russian Federation, betting is a prohibited activity, therefore all bookmaker companies must have a license to carry out the relevant activity. If the bookmaker does not have such a license, then Roskomnadzor issues a decision to block sites.
The reason why “1vin” is in no hurry to acquire a license from the Russian Federation is the introduction by law of a mandatory income tax in the form of 13% of all profits, and not only the bookmaker himself, but also his clients are required to pay the tax.
Of course, such measures can provoke an outflow of clients, because no one wants to share their honestly earned winnings, which is why companies resort to creating mirror sites. But the absence of a Russian license does not mean that the bookmaker does not have the right to carry out its activities; 1win has a foreign license that ensures security for clients.
In order to register on one of the mirrors, you must first find on the Internet one of the currently relevant mirrors. Registration is only available to adults. Registration consists of the following steps:
- you need to find and click the “Registration” field in the upper right corner
- choose the registration method that suits you (in 1 click, using social networks, using email)
In order to register in 1 click, just select your country of residence and confirm that you have read all the conditions. To register on social networks, you must select the appropriate network (Vkontakte, Odnoklassniki, Google) and confirm that you have read the agreement. To register using an email address, you must provide the following information:
- Date of Birth
- a country
- Mobile phone number
- E-mail address
- password
- repeat password
- confirm that you have read the necessary conditions
After basic registration, you need to go through the identification procedure, after which you can start replenishing your game account.
- exposure dose
- absorbed dose
- equivalent dose
- effective equivalent dose
Radioactivity
This is the ability of the nuclei of atoms of various chemical elements to be destroyed, modified with the emission of atomic and subatomic particles of high energies. During radioactive transformations, in the overwhelming majority of cases, the atomic nuclei (and therefore the atoms themselves) of some chemical elements are transformed into the atomic nuclei (atoms) of other chemical elements, or one isotope of a chemical element is transformed into another isotope of the same element.
Atoms whose nuclei are subject to radioactive decay or other radioactive transformations are called radioactive.
Isotopes
(from Greek wordsisos – “equal, identical” andtopos - "place")
These are nuclides of one chemical element, i.e. varieties of atoms of a particular element that have same atomic number but different mass numbers.
Isotopes have nuclei with the same number of protons and different numbers of neutrons and occupy the same place in the periodic table of chemical elements. There are stable isotopes, which exist unchanged indefinitely, and unstable (radioisotopes), which decay over time.
Knownabout 280 stable Andmore than 2000 radioactive isotopes116 natural and artificially obtained elements .
Nuclide (from Latinnucleus – “nucleus”) is a collection of atoms with certain values of nuclear charge and mass number.
Nuclide symbols:, WhereX – letter designation of the element,Z – number of protons (atomic number ), A – sum of the number of protons and neutrons (mass number ).
Even the very first and lightest atom in the periodic table, hydrogen, which has only one proton in its nucleus (and one electron revolves around it), has three isotopes.
Radioactive transformations
They can be natural, spontaneous (spontaneous) and artificial. Spontaneous radioactive transformations are a random, statistical process.
All radioactive transformations are usually accompanied by the release of excess energy from the nucleus of the atom in the form electromagnetic radiation.
Gamma radiation is a stream of gamma quanta with high energy and penetrating power.
X-rays are also a stream of photons - usually with lower energy. Only the “birthplace” of X-ray radiation is not the nucleus, but the electron shells. The main flux of X-ray radiation occurs in a substance when “radioactive particles” (“radioactive radiation” or “ionizing radiation”) pass through it.
The main types of radioactive transformations:
- radioactive decay;
- fission of atomic nuclei.
This is the emission, the ejection at enormous speeds from the nuclei of atoms of “elementary” (atomic, subatomic) particles, which are commonly called radioactive (ionizing) radiation.
When one isotope of a given chemical element decays, it turns into another isotope of the same element.
For natural of (natural) radionuclides, the main types of radioactive decay are alpha and beta minus decay.
Titles " alpha" And " beta” were given by Ernest Rutherford in 1900 while studying radioactive radiation.
For artificial(man-made) radionuclides, in addition, are also characterized by neutron, proton, positron (beta-plus) and rarer types of decay and nuclear transformations (mesonic, K-capture, isomeric transition, etc.).
Alpha decay
This is the emission of an alpha particle from the nucleus of an atom, which consists of 2 protons and 2 neutrons.
An alpha particle has a mass of 4 units, a charge of +2 and is the nucleus of a helium atom (4He).
As a result of the emission of an alpha particle, a new element is formed, which is located in the periodic table 2 cells to the left, since the number of protons in the nucleus, and therefore the charge of the nucleus and the element number, became two units less. And the mass of the resulting isotope turns out to be 4 units less.
A alpha – decay- this is a characteristic type of radioactive decay for natural radioactive elements of the sixth and seventh periods of the table of D.I. Mendeleev (uranium, thorium and their decay products up to and including bismuth) and especially for artificial - transuranium - elements.
That is, individual isotopes of all heavy elements, starting with bismuth, are susceptible to this type of decay.
So, for example, the alpha decay of uranium always produces thorium, the alpha decay of thorium always produces radium, the decay of radium always produces radon, then polonium, and finally lead. In this case, from a specific isotope of uranium-238, thorium-234 is formed, then radium-230, radon-226, etc.
The speed of an alpha particle when leaving the nucleus is from 12 to 20 thousand km/sec.
Beta decay
Beta decay- the most common type of radioactive decay (and radioactive transformations in general), especially among artificial radionuclides.
Each chemical element there is at least one beta-active isotope, that is, subject to beta decay.
An example of a natural beta-active radionuclide is potassium-40 (T1/2=1.3×109 years), the natural mixture of potassium isotopes contains only 0.0119%.
In addition to K-40, significant natural beta-active radionuclides are also all decay products of uranium and thorium, i.e. all elements from thallium to uranium.
Beta decay includes such types of radioactive transformations as:
– beta minus decay;
– beta plus decay;
– K-capture (electronic capture).
Beta minus decay– this is the emission of a beta minus particle from the nucleus – electron , which was formed as a result of the spontaneous transformation of one of the neutrons into a proton and an electron.
At the same time, the beta particle at speeds up to 270 thousand km/sec(9/10 the speed of light) flies out of the core. And since there are one more protons in the nucleus, the nucleus of this element turns into the nucleus of the neighboring element on the right - with a higher number.
During beta-minus decay, radioactive potassium-40 is converted into stable calcium-40 (in the next cell to the right). And radioactive calcium-47 turns into scandium-47 (also radioactive) to the right of it, which, in turn, also turns into stable titanium-47 through beta-minus decay.
Beta plus decay– emission of beta-plus particles from the nucleus – positron (a positively charged “electron”), which was formed as a result of the spontaneous transformation of one of the protons into a neutron and a positron.
As a result of this (since there are fewer protons), this element turns into the one next to it on the left in the periodic table.
For example, during beta-plus decay, the radioactive isotope of magnesium, magnesium-23, turns into a stable isotope of sodium (on the left) - sodium-23, and the radioactive isotope of europium - europium-150 turns into a stable isotope of samarium - samarium-150.
– emission of a neutron from the nucleus of an atom. Characteristic of nuclides of artificial origin.
When a neutron is emitted, one isotope of a given chemical element transforms into another, with less weight. For example, during neutron decay, the radioactive isotope of lithium, lithium-9, turns into lithium-8, radioactive helium-5 into stable helium-4.
If a stable isotope of iodine - iodine-127 - is irradiated with gamma rays, then it becomes radioactive, emits a neutron and turns into another, also radioactive isotope - iodine-126. That's an example artificial neutron decay .
As a result of radioactive transformations, they can form isotopes of other chemical elements or the same element, which may themselves be radioactive elements.
Those. the decay of a certain initial radioactive isotope can lead to a certain number of successive radioactive transformations of various isotopes of different chemical elements, forming the so-called. "decay chains".
For example, thorium-234, formed during the alpha decay of uranium-238, turns into protactinium-234, which in turn turns back into uranium, but into a different isotope - uranium-234.
All these alpha and beta minus transitions end with the formation of stable lead-206. And uranium-234 undergoes alpha decay - again into thorium (thorium-230). Further, thorium-230 by alpha decay - into radium-226, radium - into radon.
Fission of atomic nuclei
Is it spontaneous, or under the influence of neutrons, core splitting atom into 2 approximately equal parts, into two “shards”.
When dividing they fly out 2-3 extra neutrons and an excess of energy is released in the form of gamma quanta, much greater than during radioactive decay.
If for one act of radioactive decay there is usually one gamma ray, then for 1 act of fission there are 8 -10 gamma quanta!
In addition, flying fragments have high kinetic energy (speed), which turns into thermal energy.
Departed neutrons can cause fission two or three similar nuclei, if they are nearby and if neutrons hit them.
Thus, it becomes possible to implement a branching, accelerating fission chain reaction atomic nuclei releasing enormous amounts of energy.
Fission chain reaction
If the chain reaction is allowed to develop uncontrollably, an atomic (nuclear) explosion will occur.
If the chain reaction is kept under control, its development is controlled, not allowed to accelerate and constantly withdraw released energy(heat), then this energy (“ atomic energy") can be used to generate electricity. This is done in nuclear reactors and nuclear power plants.
Characteristics of radioactive transformations
Half life (T1/2 ) – the time during which half of the radioactive atoms decay and their the quantity is reduced by 2 times.
The half-lives of all radionuclides are different - from fractions of a second (short-lived radionuclides) to billions of years (long-lived).
Activity– this is the number of decay events (in general, acts of radioactive, nuclear transformations) per unit of time (usually per second). The units of activity are becquerel and curie.
Becquerel (Bq)– this is one decay event per second (1 disintegration/sec).
Curie (Ci)– 3.7×1010 Bq (disp./sec).
The unit arose historically: 1 gram of radium-226 in equilibrium with its daughter decay products has such activity. It was with radium-226 that the Nobel Prize laureates, the French scientific spouses Pierre Curie and Marie Sklodowska-Curie, worked for many years.
Law of Radioactive Decay
The change in the activity of a nuclide in a source over time depends on the half-life of a given nuclide according to an exponential law:
AAnd(t) = AAnd (0) × exp(-0.693t/T1/2 ),
Where AAnd(0) – initial activity of the nuclide;
AAnd(t) – activity after time t;
T1/2 – half-life of the nuclide.
Relationship between mass radionuclide(without taking into account the mass of the inactive isotope) and his activity is expressed by the following relationship:
Where mAnd– radionuclide mass, g;
T1/2 – half-life of the radionuclide, s;
AAnd– radionuclide activity, Bq;
A– atomic mass of the radionuclide.
Penetrating power of radioactive radiation.
Alpha particle range depends on the initial energy and usually ranges from 3 to 7 (rarely up to 13) cm in air, and in dense media it is hundredths of a mm (in glass - 0.04 mm).
Alpha radiation does not penetrate a sheet of paper or human skin. Due to their mass and charge, alpha particles have the greatest ionizing ability; they destroy everything in their path, therefore alpha-active radionuclides are the most dangerous for humans and animals when ingested.
Beta particle range in the substance due to its low mass (~ 7000 times
Less than the mass of the alpha particle), the charge and size are much larger. In this case, the path of a beta particle in matter is not linear. Penetration is also dependent on energy.
The penetrating ability of beta particles formed during radioactive decay is in the air reaches 2÷3 m, in water and other liquids it is measured in centimeters, in solids - in fractions of cm.
Beta radiation penetrates into body tissue to a depth of 1÷2 cm.
Attenuation factor for n- and gamma radiation.
The most penetrating types of radiation are neutron and gamma radiation. Their range in the air can reach tens and hundreds of meters(also depending on energy), but with less ionizing power.
As protection against n- and gamma radiation, thick layers of concrete, lead, steel, etc. are used, and we are talking about the attenuation factor.
In relation to the cobalt-60 isotope (E = 1.17 and 1.33 MeV), for a 10-fold attenuation of gamma radiation, protection is required from:
- lead about 5 cm thick;
- concrete about 33 cm;
- water – 70 cm.
For 100-fold attenuation of gamma radiation, 9.5 cm thick lead shielding is required; concrete – 55 cm; water – 115 cm.
Units of measurement in dosimetry
Dose (from Greek - “share, portion”) irradiation.
Exposure dose(for X-ray and gamma radiation) – determined by air ionization.
SI unit of measurement – “coulomb per kg” (C/kg)- this is the exposure dose of x-ray or gamma radiation, when created in 1 kg dry air, a charge of ions of the same sign is formed, equal to 1 Cl.
The non-system unit of measurement is "x-ray".
1 R = 2.58× 10 -4 Kl/kg.
A-priory 1 roentgen (1P)– this is the exposure dose upon absorption of which 1 cm3 dry air is formed 2,08 × 10 9 ion pairs.
The relationship between these two units is as follows:
1 C/kg = 3.68 ·103 R.
Exposure dose 1Р corresponds to the absorbed dose in the air 0.88 rad.
Dose
Absorbed dose– the energy of ionizing radiation absorbed by a unit mass of matter.
The radiation energy transferred to a substance is understood as the difference between the total kinetic energy of all particles and photons entering the volume of matter under consideration and the total kinetic energy of all particles and photons leaving this volume. Therefore, the absorbed dose takes into account all the ionizing radiation energy left within that volume, regardless of how that energy is spent.
Absorbed dose units:
Gray (Gr)– unit of absorbed dose in the SI system of units. Corresponds to 1 J of radiation energy absorbed by 1 kg of substance.
Glad– extra-systemic unit of absorbed dose. Corresponds to a radiation energy of 100 erg absorbed by a substance weighing 1 gram.
1 rad = 100 erg/g = 0.01 J/kg = 0.01 Gy.
The biological effect at the same absorbed dose is different for different types of radiation.
For example, with the same absorbed dose alpha radiation turns out much more dangerous than photon or beta radiation. This is due to the fact that alpha particles create denser ionization along their path in biological tissue, thus concentrating the harmful effects on the body in a specific organ. In this case, the entire body experiences a much greater inhibitory effect of radiation.
Consequently, to create the same biological effect when irradiated with heavy charged particles, a lower absorbed dose is required than when irradiated with light particles or photons.
Equivalent dose– product of the absorbed dose and the radiation quality factor.
Equivalent dose units:
sievert(Sv) is a unit of measurement for dose equivalent, any type of radiation that produces the same biological effect as the absorbed dose in 1 Gy
Hence, 1 Sv = 1 J/kg.
Bare(non-systemic unit) is the amount of energy of ionizing radiation absorbed 1 kg biological tissue, in which the same biological effect is observed as with the absorbed dose 1 rad X-ray or gamma radiation.
1 rem = 0.01 Sv = 100 erg/g.
The name “rem” is formed from the first letters of the phrase “biological equivalent of an x-ray.”
Until recently, when calculating the equivalent dose, “ radiation quality factors » (K) – correction factors that take into account the different effects on biological objects (different abilities to damage body tissues) of different radiations at the same absorbed dose.
Now these coefficients in the Radiation Safety Standards (NRB-99) are called “weighting coefficients for individual types of radiation when calculating the equivalent dose (WR).”
Their values are respectively:
- X-ray, gamma, beta radiation, electrons and positrons – 1 ;
- protons with E more than 2 MeV – 5 ;
- neutrons with E less than 10 keV) – 5 ;
- neutrons with E from 10 kev to 100 kev – 10 ;
- alpha particles, fission fragments, heavy nuclei – 20 etc.
Effective equivalent dose– equivalent dose, calculated taking into account the different sensitivity of different body tissues to radiation; equal to equivalent dose, obtained by a specific organ, tissue (taking into account their weight), multiplied by corresponding " radiation risk coefficient ».
These coefficients are used in radiation protection to take into account the different sensitivity of different organs and tissues in the occurrence of stochastic effects from exposure to radiation.
In NRB-99 they are called “weighing coefficients for tissues and organs when calculating the effective dose.”
For the body as a whole this coefficient is taken equal to 1 , and for some organs it has the following meanings:
- bone marrow (red) – 0.12; gonads (ovaries, testes) – 0.20;
- thyroid gland – 0.05; leather – 0.01, etc.
- lungs, stomach, large intestine – 0.12.
To evaluate the full effective equivalent dose received by a person, the indicated doses for all organs are calculated and summed up.
To measure equivalent and effective equivalent doses, the SI system uses the same unit - sievert(Sv).
1 Sv equal to the equivalent dose at which the product of the absorbed dose in Gr eyah (in biological tissue) by the weighting coefficients will be equal to 1 J/kg.
In other words, this is the absorbed dose at which 1 kg substances release energy into 1 J.
The non-systemic unit is the rem.
Relationship between units of measurement:
1 Sv = 1 Gy * K = 1 J/kg * K = 100 rad * K = 100 rem
At K=1(for x-rays, gamma, beta radiation, electrons and positrons) 1 Sv corresponds to the absorbed dose in 1 Gy:
1 Sv = 1 Gy = 1 J/kg = 100 rad = 100 rem.
Back in the 50s, it was established that with an exposure dose of 1 roentgen, air absorbs approximately the same amount of energy as biological tissue.
Therefore, it turns out that when estimating doses we can assume (with minimal error) that exposure dose of 1 roentgen for biological tissue corresponds(equivalent) absorbed dose of 1 rad And equivalent dose of 1 rem(at K=1), that is, roughly speaking, 1 R, 1 rad and 1 rem are the same thing.
With an exposure dose of 12 μR/hour per year, we receive a dose of 1 mSv.
In addition, to assess the impact of AI, the following concepts are used:
Dose rate– dose received per unit of time (second, hour).
Background– the exposure dose rate of ionizing radiation in a given location.
Natural background– the exposure dose rate of ionizing radiation created by all natural sources of radiation.
Sources of radionuclides entering the environment
1. Natural radionuclides, which have survived to our time from the moment of their formation (possibly from the time of the formation of the solar system or the Universe), since they have long half-lives, which means a long lifetime.
2.Radionuclides of fragmentation origin, which are formed as a result of the fission of atomic nuclei. They are formed in nuclear reactors in which a controlled chain reaction occurs, as well as during testing of nuclear weapons (uncontrolled chain reaction).
3. Radionuclides of activation origin are formed from ordinary stable isotopes as a result of activation, that is, when a subatomic particle (usually a neutron) enters the nucleus of a stable atom, as a result of which the stable atom becomes radioactive. They are obtained by activating stable isotopes by placing them in the reactor core, or by bombarding a stable isotope in particle accelerators with protons, electrons, etc.
Areas of application of radionuclide sources
AI sources find applications in industry, agriculture, scientific research and medicine. In medicine alone, approximately one hundred isotopes are used for various medical research, diagnosis, sterilization and radiotherapy.
Around the world, many laboratories use radioactive materials for scientific research. Thermoelectric generators based on radioisotopes are used to produce electricity for autonomous power supply of various equipment in remote and hard-to-reach areas (radio and light beacons, weather stations).
Everywhere in industry, instruments containing radioactive sources are used to monitor technological processes (density, level and thickness gauges), non-destructive testing instruments (gamma flaw detectors), and instruments for analyzing the composition of matter. Radiation is used to increase the size and quality of crops.
The influence of radiation on the human body. Effects of radiation
Radioactive particles, possessing enormous energy and speed, when passing through any substance they collide with atoms and molecules of this substance and lead to their destruction ionization, to the formation of “hot” ions and free radicals.
Since biological Human tissue is 70% water, then to a large extent It is water that undergoes ionization. Ions and free radicals form compounds harmful to the body, which trigger a whole chain of sequential biochemical reactions and gradually lead to the destruction of cell membranes (cell walls and other structures).
Radiation affects people differently depending on gender and age, the state of the body, its immune system, etc., but especially strongly on infants, children and adolescents. When exposed to radiation hidden (incubation, latent) period, that is, the delay time before the onset of a visible effect can last for years or even decades.
The impact of radiation on the human body and biological objects causes three different negative effects:
- genetic effect for hereditary (sex) cells of the body. It can and does manifest itself only in posterity;
- genetic-stochastic effect, manifested for the hereditary apparatus of somatic cells - body cells. It manifests itself during the life of a particular person in the form of various mutations and diseases (including cancer);
- somatic effect, or rather, immune. This is a weakening of the body’s defenses and immune system due to the destruction of cell membranes and other structures.
Related materials

Beta decay is the spontaneous transformation of a nucleus (A,Z) into an isobar nucleus (A,Z +
1) as a result of the emission of leptons (electron and antineutrino, positron and neutrino), or the absorption of an electron with the emission of neutrinos (e-capture).
During the decay process, energy is released
where M at - masses of atoms. (Here we neglected the difference in the binding energies of electrons in the initial and final atoms.) The energy released as a result of β decay is mainly carried away by light particles - leptons (electron, electron antineutrino, positron, electron neutrino).
β-decay energies vary from 0.02 MeV
3 H → 3 He + e − + e + 0.02 MeV
11 Li → 11 Be + e − + e + 20.4 MeV
Half-lives also vary over a wide range from 10 -3 s to 10 16 years. The long lifetimes of β-radioactive nuclei are explained by the fact that β decay occurs as a result of weak interaction.
Nuclei that undergo beta decay are located throughout the periodic table of elements. From the Weizsäcker formula for nuclear binding energy
Since A = N + Z, formula (2) determines the relationship between the number of protons Z and neutrons N for nuclei of the stability valley. At Z< Z равн
ядро нестабильно к β - -распаду, а при Z >Z is equal to β+ -decay and E-capture. For all A, β-stable nuclei should cluster around values of Z equal. From (2) it is clear that for small A Z is equal to ~
A/2 i.e. stable light nuclei should have approximately the same number of protons and neutrons (the role of Coulomb energy is small). As A increases, the role of Coulomb energy increases, and the number of neutrons in stable nuclei begins to exceed the number of protons. The left side of Fig. 1 shows the mass parabola for nuclei with odd A = 125. The stable 125 Te nucleus is at the minimum of the mass parabola (respectively, at the maximum of the parabola for binding energy). 125 In, 125 Sn, 125 Sb are subject to β - decay, 125 I, 125 Xe, 125 Cs, 125 Ba - β + decay. The greater the beta decay energy of nuclei (the mass difference between neighboring isobars), the further they are from the line of stability.
For even A, instead of one parabola, due to the pairing energy (the last term in formula (1)), two parabolas are obtained (the right side of Fig. 1): for odd-odd nuclei and for even-even nuclei. Despite the fact that the pairing energy is small compared to the total binding energy of the nucleus (for nuclei with A ~ 100 the binding energy is about 1000 MeV, the distance between the parabolas is about 2 MeV), this leads to important consequences. Some odd-odd nuclei (for example 128 I) can experience both β - decay and β + decay and e-capture. There are significantly more stable even-even nuclei than stable nuclei with odd A and, even more so, than stable odd-odd nuclei, of which there are only four (2 H, 6 Li, 10 B, 14 N). For a given A, there can be several stable even-even nuclei (for example, 136 Xe, 136 Ba, 136 Ce). Elements with odd Z rarely have more than one stable isotope, while for elements with even Z it is not uncommon (112 Sn, 114 Sn, 115 Sn, 116 Sn, 117 Sn, 118 Sn, 119 Sn, 120 Sn, 122 Sn , 124 Sn). In some cases, when beta decay into an odd-odd nucleus is impossible for even-even nuclei, a transition with a change in Z by two units turns out to be energetically possible - double beta decay. 128 Te and 130 Te undergo such exotic decay. Their content in the natural mixture of this element is 31.7% and 33.8%, respectively. The probability of double beta decay is very small, half-lives T 1/2 (128 Te) = 7.7 10 28 years,
T 1/2 (130 Te) = 2.7·10 21 years.
As a result of beta decay, three particles are formed: a finite nucleus and a pair of leptons. The energy imparted to the nucleus due to its large mass is small and can be neglected. Therefore, the kinetic energy released during beta decay is almost entirely carried away by a pair of leptons, and the energy distribution between them can be arbitrary. Thus, the energy spectrum of positrons (electrons) and neutrinos (antineutrinos) must be continuous in the range from 0 to Q b (see Fig. 2).
In the case of capture of an orbital electron by a nucleus, two products are formed: the final nucleus and a neutrino. The energy distribution between them is therefore unambiguous, and almost all of it is carried away by neutrinos. Thus, the spectrum of neutrinos during e-capture for fixed states of the initial and final nuclei will be monochromatic, in contrast to beta decay. The e-capture involves mainly the electrons of the shells closest to the nucleus (primarily the K-shell). For such electrons, the probability of being inside the nucleus is greatest.
A characteristic feature of all types of beta decay is the participation of neutrinos or antineutrinos. For the first time, the hypothesis of the existence of neutrinos was put forward by Pauli in 1930 to “save” the laws of conservation of energy and angular momentum. The continuous nature of the spectrum of electrons (positrons) could not be explained without abandoning the law of conservation of energy. The neutrino hypothesis made it possible not to abandon such a fundamental principle. Many years passed before Cowen and Reines managed to detect an electron antineutrino.
Beta decay occurs as a result of weak interactions. In Fig. Figure 3 shows the Feynman diagram for β - -decay. At the quark level, beta decay occurs when a d-quark transforms into a u-quark or vice versa. At the nucleon level, this corresponds to the transitions of a neutron into a proton or a proton into a neutron. Moreover, if a neutron can transform into a proton in a free state, then the reverse transition is possible only for protons in the nucleus.
Beta decays are divided into allowed and forbidden, differing in transition probabilities. Allowed transitions include transitions in which the total orbital momentum l carried away by the electron and neutrino is equal to zero. Forbidden transitions are divided according to the order of prohibition, which is determined by the orbital momentum l. If l = 1, then this is a forbidden transition of the first order, l min = 2 - of the second order, etc. All other things being equal, the ratio of the probabilities of the departure of a particle with orbital angular moments l = 0 (w 0) and l ≠0 (w l)
w l /w 0 ~ (R/) 2l, |
where R is the radius of the nucleus, and is the wavelength.
Beta decays are also divided into transitions of the Fermi type, in which the spins of the emitted leptons are antiparallel, and of the Gamow–Teller type, in which the spins of the emitted leptons are parallel.
How can one understand such a strong dependence of the probability of beta transitions on the orbital angular momentum of the escaping leptons?
 A particle with momentum p and impact parameter b hits a nucleus with radius R. The classical angular momentum pb is equal to the orbital momentum
A particle with momentum p and impact parameter b hits a nucleus with radius R. The classical angular momentum pb is equal to the orbital momentum
Let us estimate at what l condition (5) is satisfied. The radii of even the heaviest nuclei are less than 10 fm. To estimate, let's set the radius to be 10 fm and the beta decay energy to be 20 MeV. Then for electrons we can use the ultrarelativistic approximation and rewrite (5) in the form
From (7) it is clear that the orbital momentum of leptons emitted during beta decay can only be zero in a semiclassical analysis, and transitions with l ≠0 are prohibited. However, the quantum properties of particles mean that such forbidden transitions occur, although they are greatly suppressed. Moreover, the smaller the ratio R/, the stronger it is. The probability of a beta transition is proportional to (R/) 2l. Since during beta decay R<< и более того
R + а << ,
где a - ширина кулоновского барьера, он практически не влияет на вероятность
бета-распада, так как образовавшиеся электроны (позитроны) сразу имеют ненулевую
вероятность нахождения вне ядра. Влияние кулоновских сил сводится к тому, что
вылетевшие электроны тормозятся, а позитроны ускоряются кулоновским полем ядра,
что приводит к изменению формы их спектров.
The foundations of the theory of weak interactions and β-decay were laid by Fermi in 1934. By 1958, this theory was generalized into the universal four-fermion theory of weak interactions, according to which the elementary process of weak interaction is a local interaction of four fermions, i.e. particles with half-integer spins. Currently, the processes of both weak and electromagnetic interactions are explained in a new theory - the unified theory of electroweak interactions. According to this theory, the weak interaction occurs through the exchange of virtual intermediate bosons. Fermi's theory assumed that the interaction that leads to beta decay is small compared to the interaction that forms the states of the nucleus. This made it possible to use perturbation theory and write down the decay probability per unit time in the form (Fermi’s golden rule)
where M fi is the matrix element of beta decay, ρ f (E) is the density of final states.
V fi is the Hamiltonian of the weak interaction, ψ i and ψ* f are the wave functions of the initial and final states of the system.
In the initial state there is a nucleus described by the wave function i, and in the final state there is a nucleus, electron and antineutrino, described by the wave functions φ f, φ e, φ ν. Assuming that the final nucleus, electron and antineutrino do not interact with each other, we obtain the following expression for the wave function of the final state of the system: ψ f = φ f φ e φ ν .
In this case, the matrix element of beta decay has the form
where G F is the Fermi constant of weak interaction.
If we neglect the interaction of the electron and antineutrino with surrounding particles, then plane waves can be chosen as their wave functions:
where p and q are the electron and neutrino momenta. Neglecting the recoil energy of the nucleus, we write
Q b = T e + , dQ b = dT e = d,
where T e and are the kinetic energies of the electron and neutrino. Assuming the neutrino mass equal to zero, we can write
where T e is the kinetic energy of the electron. The distribution of the number of electrons depending on their energy has the form:
describing beta decay. It should be noted that the beta spectrum is distorted by the Coulomb field of the atom, which consists of the field of the nucleus and the electron shell. Therefore, a factor F(T e ,Z) has been added to expression (17), which is defined as the ratio of the probability of finding an electron at a certain point taking into account the atomic field (Z = 0) to the probability without taking the field into account (Z = 0). The distortion introduced into the beta spectrum by the Coulomb field of the atom is especially significant at the beginning of the spectrum, that is, for particles with low energy. In this case, the center of gravity of the distribution curve shifts towards low energies for electrons and high energies for positrons (Fig. 4). This displacement is greater, the greater the nuclear charge.
Relation (17) was obtained under the assumption that the neutrino mass = 0. In this case, in the high-energy part of the electron spectrum dN e /dT e 0. However, if 0 instead of (15b) for the end of the electron spectrum, when the neutrino energy is low, we need to write
This dependence of probability on energy release is characteristic not only of beta decay, but also of other weak decays and is called Sargent's rules
.
The characteristic momenta of leptons during beta decay are such that the relation
i.e., to an expression that depends only on the states of the initial and final nuclei and does not depend on the momenta of leptons. The shape of the beta spectrum in this case is determined only by the density of the final states. These are allowed beta transitions. If the matrix element = 0 in (18), then you need to expand the exponential into a series according to the power of the exponent. The degree of the first term of this series, which makes a non-zero contribution to the matrix element, determines the order of prohibition of transition. From relation (22) it follows that the probability of a β transition should decrease by approximately 10 4 with an increase in the prohibition order by 1.
At allowed transitions
The intersection of the linear function f(T e) with the abscissa axis determines the energy of beta decay - Q b.
Gamow-Teller transitions are not taken into account in Fermi theory, since in it the matrix element (10) is replaced by the matrix element (11). These transitions arise only when terms that change the spin states of the particles are introduced into the weak interaction Hamiltonian V fi.
For allowed transitions, l = 0. In this case, the wave functions of leptons are spherically symmetric and therefore leptons are emitted in different directions with the same probability. For forbidden transitions, the wave functions of leptons are no longer spherically symmetric, due to which the probability of their escape in some directions is greatly suppressed.
The selection rules for total momentum and parity in the case of beta decay can be written as