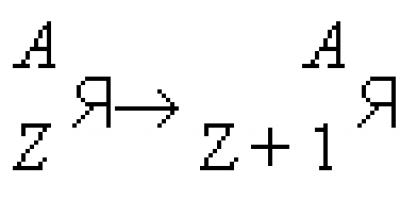§ 8. Isotopes (continued)
Isotopes of chlorine are written as follows:
Most of the chemical properties of the isotopes of chlorine, as well as, for example, potassium (K and K) or argon (Ar and Ar), as well as the isotopes of many other chemical elements, are practically the same. Only hydrogen isotopes, due to a sharp multiple increase in their relative atomic mass, have differences in chemical properties. They were even given individual names and chemical symbols: protium - H; deuterium - H, or D; tritium - H, or T (Fig. 37). We can now give a modern, more rigorous and scientific definition of a chemical element.

1. Refer to the electronic application. Study the lesson material and complete the assigned tasks.
2. Find email addresses on the Internet that can serve as additional sources that reveal the content of keywords and phrases in the paragraph. Offer your help to the teacher in preparing a new lesson - make a report on the key words and phrases of the next paragraph.
1. Why do you think the isotopes of potassium K and argon Ar, which have the same masses, exhibit different properties?
2. Why in D.I. Mendeleev’s table the relative atomic mass of argon is close to 40, and potassium is close to 39?
3. Using the names of the elementary particles that make up atomic nuclei, give another definition of isotopes.
4. Why are chlorine isotopes identical in their properties, while the properties of hydrogen isotopes are different?
5. Why is deuterium water D 2 O called heavy water? Prepare the message “Comparison of the properties of light and heavy water” using additional sources of information.
6. Find in D.I. Mendeleev’s table three pairs of elements in which, like the Ar - K pair, the element with a large relative atomic mass is located first.
>> Atoms. Ions. Chemical elements. For the curious. Chemical elements in living nature
Atoms. Ions. Chemical elements
The material in this paragraph will help you:
> find out what structure it has atom;
> understand the difference between an atom and an ion;
> learn the names and designations of chemical elements - certain types of atoms;
> use the periodic system of D.I. Mendeleev as a source of information about chemical elements.
Atoms.
Ancient Greek philosophers thought about substances and their structure. They claimed that substances consist of atoms - invisible and indivisible particles, and as a result of their connection, the surrounding world was formed and exists.
1 A filter at home can be cotton wool or a bandage folded several times. The filter must be placed in a household watering can.
Translated from Greek, the word “atom” means “indivisible.”
The existence of atoms was proven only in the 19th century. using complex physical experiments. At the same time, it was established that the atom is not a continuous, monolithic particle. It consists of a nucleus and electrons. In 1911, one of the first models of the atom was proposed - planetary. According to this model, the nucleus is located in the center of the atom and occupies a small part of its volume, and electrons move around the nucleus in certain orbits, like planets around the Sun (Fig. 32).
An electron is thousands of times smaller than an atomic nucleus. This is a negatively charged particle. Its charge is the smallest that exists in nature. Therefore, the magnitude of the electron charge physicists taken as a unit of measurement for the charges of the smallest particles (besides electrons, there are other particles). Thus, the charge of the electron is - 1. This particle is designated as follows: .
The nucleus of an atom is positively charged. The charge of the nucleus and the total charge of all the electrons of the atom are equal in magnitude, but opposite in sign. Therefore the atom is electrically neutral. If the charge of the nucleus of an atom is +1, then such an atom contains one electron, if +2 - two electrons, etc.

Rice. 32. Structure of the simplest atom (planetary model)
An atom is the smallest electrically neutral particle of matter, consisting of a positively charged nucleus and negatively charged electrons that move around it.
Ions.
An atom under certain conditions can lose or gain one or more electrons. At the same time, it becomes a positively or negatively charged particle - an ion 1.
An ion is a charged particle formed as a result of the loss or gain of one or more electrons by an atom.
1 The word “ion” in Greek means “going.” Unlike an electrically neutral atom, an ion is capable of moving in an electric field.
If an atom loses one electron, then an ion with a charge of +1 is formed, and if it gains an electron, then the charge of the ion will be equal to - I (Scheme 5). If an atom loses or gains two
electrons, ions are formed with charges +2 or -2, respectively.

Scheme 5. Formation of ions from atoms
There are also ions formed from several atoms.
Chemical elements.
There are an infinite number of atoms in the Universe. They are distinguished by the charges of their nuclei.
A type of atom with a certain nuclear charge is called a chemical element.
Atoms with a nuclear charge of +1 belong to one chemical element, with a charge of +2 to another element, etc.
Currently, 115 chemical elements are known. The nuclear charges of their atoms range from +1 to +112, as well as +114, +116 and +118.
Almost 90 elements exist in nature, and the rest (usually those with the highest atomic nuclei charges) are man-made elements. They are obtained by scientists using unique research equipment. The nuclei of atoms of artificial elements are unstable and quickly decay.
Names of chemical elements, atoms and ions.
Each chemical element has a name. Modern names of elements come from Latin names (Table I). They are always written with a capital letter.
Table I 
Until recently, 18 elements had other (traditional) names, which can be found in previously published chemistry textbooks, as well as in Table I. For example, the traditional name of one of these elements is hydrogen, and the modern name is Hydrogen.
The names of the elements are also used for the corresponding particles: Hydrogen atom ( hydrogen), Hydrogen (hydrogen) ion.
You will learn the names of ions formed from several atoms later.
The names of chemical elements have different origins. Some are associated with the names or properties (color, smell) of substances, others with the names of planets, countries, etc. There are elements named after outstanding scientists. The origin of some names is unknown because they arose a long time ago.
This is interesting
The modern name of one of the elements is Mercury. It differs from the Latin name (Hydrargyrum), but is close to the English (Mercury) and French (Mercure).
What do you think about the origin of the names of such elements: Europium, Francium, Neptunium, Promethium, Mendelevium?
This is interesting
The symbols of the elements are the same in all countries.
Symbols of chemical elements.
Each element, in addition to the name, also has an abbreviated designation - a symbol or sign. Nowadays, they use the symbols of the elements proposed almost 200 years ago by the famous Swedish chemist J. J. Berzelius (1779-1848). They consist of one Latin letter (the first in the Latin names of elements) or two1. In Table I, such letters are highlighted in italics in the element names.

Rice. 33. Cell of the periodic table
The pronunciation of the symbols of almost all elements coincides with their names. For example, the symbol for the element Iodine I is read “iod” rather than “i”, and the element symbol Ferrum Fe is read “ferum” rather than “fe”. All exceptions are collected in Table I.
In some cases, the general designation of a chemical element is used - E.
The symbols and names of chemical elements are contained in the periodic system of D.I. Mendeleev.
Periodic table of chemical elements by D. I. Mendeleev .
In 1869, the Russian chemist Dmitry Ivanovich Mendeleev proposed a table in which he placed the 63 elements known at that time. This table was called the periodic system of chemical elements.
Our textbook contains two versions of it: short (endpaper I) and long (endpaper II).
The periodic table has horizontal rows called periods and vertical columns called groups. Intersecting, they form cells that contain the most important information about chemical elements.
Each cell is numbered. It contains the symbol of the element, and under it - the name (Fig. 33).
1 The symbols of the four recently discovered elements consist of three letters.
Dmitry Ivanovich Mendeleev (1834-1907)

An outstanding chemist, member and honorary member of the academies of sciences of many countries. In 1869, at the age of 35, he created the periodic table (system) of chemical elements and discovered the periodic law - the fundamental law of chemistry. Based on the periodic law, he outlined chemistry in his textbook “Fundamentals of Chemistry,” which was reprinted many times in Russia and other countries. Conducted thorough studies of solutions and developed a theory of their structure (1865-1887). Deduced the general equation of gas state (1874). He proposed a theory of the origin of oil, developed a technology for the production of smokeless gunpowder, and made a significant contribution to the development of the science of measurements - metrology.
The cell number is called the serial number of the element placed in it. Its general designation is Z. The expression “the serial number of the element Neon is 10” is abbreviated as follows: Z(Ne) = 10. The serial number of an element coincides with the charge of the nucleus of its atom and the number of electrons in it.
In the periodic table, all elements are arranged in order of increasing charge of the atomic nuclei.
So, from the periodic table of D.I. Mendeleev, you can obtain the following information about a chemical element:
Symbol;
Name;
serial number;
charge of the nucleus of an atom;
number of electrons in an atom;
number of the period in which the element is located;
number of the group in which it is placed.
Find an element with serial number 5 in the periodic table and write down information about it in your notebook.
Prevalence of chemical elements.
Some elements are found in nature “at every step,” while others are extremely rare. The abundance of an element in air, water, soil, etc. is assessed by comparing the number of its atoms with the number of atoms of other elements.
Vladimir Ivanovich Vernadsky (1863-1945)

Russian and Ukrainian natural scientist, academician of the USSR Academy of Sciences and the Ukrainian Academy of Sciences, first president of the Ukrainian Academy of Sciences (1919). One of the founders of geochemistry. He put forward a theory of the origin of minerals. He studied the role of living organisms in geochemical processes. Developed the doctrine of the biosphere and noosphere. He studied the chemical composition of the lithosphere, hydrosphere, and atmosphere. Organizer of several research centers. Founder of the school of geochemical scientists.
The distribution of elements in different parts of our planet is studied by the science of geochemistry. The outstanding Russian scientist V.I. Vernadsky made a significant contribution to its development.
The atmosphere consists almost entirely of two gases - nitrogen and oxygen. There are four times more nitrogen molecules in the air than molecules oxygen Therefore, the first place in prevalence in the atmosphere is occupied by the element Nitrogen, and the second place by Oxygen.
The hydrosphere is rivers, lakes, seas, oceans in which small amounts of solids and gases. Taking into account the composition of the water molecule, it is easy to come to the conclusion that the hydrosphere contains the most hydrogen atoms.
The lithosphere, or earth's crust, is the solid surface layer of the Earth. It contains many elements. The most common are Oxygen (58% of all atoms), Silicon (19.6%) and Aluminum (6.4%).
The same elements exist in the Universe as on our planet. The first and second places in abundance in it are occupied by Hydrogen (92% of all atoms) and Helium (7%) - elements whose atoms have the simplest structure.
conclusions
An atom is the smallest electrically neutral particle of a substance, which consists of a positively charged nucleus and negatively charged electrons.
An ion is a positively or negatively charged particle formed as a result of the loss or addition of one or more electrons by an atom.
A type of atom with a certain nuclear charge is called a chemical element. Each element has a name and symbol.
The most important information about chemical elements is contained in the periodic table created by the Russian scientist D.I. Mendeleev.
Almost 90 chemical elements exist in nature; they vary in prevalence.
?
37. Describe the structure of an atom.
38. Define an ion. How is this particle formed from an atom?
39. What is a chemical element? Why can’t it be identified with an atom or substance?
40. Does one element transform into another if an atom loses (gains) an electron? Explain your answer.
41. Find and read the following symbols of chemical elements in the periodic table: Li, H, Al, 0, C, Na, S, Cu, Ag, N, Au. Name these elements.
42. Which symbol corresponds to Ferrum (F, Fr, Fe), Silicium (C, Cl, S, Si, Sc), Carbon (K, C, Co, Ca, Cr, Kr)?
43. Write down from the periodic table the symbols of all elements that begin with the letter A. How many such elements are there?
44. Prepare a short report on the origin of the names of Hydrogen, Helium or any other element.
45. Fill in the blanks: a) Z(...) = 8, Z(...) = 12; b) Z(C) = ..., Z(Na) = ...
46. Fill out the table:
47. Using the data given in the text of the paragraph, determine approximately how many Oxygen atoms in the earth’s crust there are per I atom of Silicon and per I atom of Aluminum.
For the curious
Chemical elements in living nature It is estimated that on average 80% of the mass of plants is water. This substance also predominates in animal and human organisms. Consequently, the most common element in living nature, as well as in the hydrosphere, is Hydrogen.

Rice. 34. Chemical elements in the adult body (as a percentage of the total number of atoms)
The human body requires more than 20 chemical elements. They are called bioelements (Fig. 34). They are found in air, water, and many substances that enter the body with food. Carbon, Oxygen, Hydrogen, Nitrogen, Sulfur are found in proteins and other substances that make up the body. Potassium and Sodium are found in blood, cellular fluids, etc. Oxygen, Phosphorus and Calcium are essential for bone formation. Ferrum, Fluor, Iodine are very important for humans. A lack of Ferrum in the body leads to anemia, Fluor causes caries, and Iodine slows down the mental development of a child.
We are fascinated by the poetic names of elementary particles, but at the same time, their diversity causes us a quiet sadness: what a pity that the world is not as simple and understandable as Democritus of Abdera imagined it. The main achievement of this ancient Greek philosopher (about 460 BC - about 370 BC) was the development of the doctrine of Leucippus about the “atom” - an indivisible particle of matter with true existence, neither destroyed nor created ( atomistic materialism). Democritus described the world as a system of atoms in the void, rejecting the infinite divisibility of matter. Atoms, according to this theory, move in empty space (the Great Emptiness, as Democritus said) chaotically, collide and, due to the correspondence of shapes, sizes, positions and orders, either stick together or fly apart. The resulting compounds are held together and thus complex bodies arise. Movement itself is a property naturally inherent in atoms. Bodies are combinations of atoms. The diversity of bodies is due both to the difference in the atoms composing them and to the difference in the order of assembly, just as different words are formed from the same letters.
Democritus called his particle “atom,” i.e., “indivisible.” Modern scientists are also able to come up with names for elementary particles.
Boson
A class of particles often associated with forces, i.e. they act as force carriers. Subject to Bose-Einstein statistics and named after the Indian physicist Shatyendranath Bose (1894-1974). The suffix “-on” is Greek; a hundred years ago it began to be constantly used to form the names of newly discovered particles.
Fermion
This is also a whole class of particles, but they, unlike bosons, obey different statistics, namely Fermi-Dirac statistics, and are usually associated not with force, but with matter. Named in honor of the American physicist of Italian origin Enrico Fermi (1901-1954), who, along with Robert Oppenheimer, is considered one of the fathers of the atomic bomb.
Gluon
A type of boson responsible for the strong interaction between quarks. When “naming” these particles in 1962, Murray Gell-Mann dispensed with the Greek language, but used the ordinary English word “glue”, since they seem to glue pairs of quarks into mesons, and triplets of quarks into protons and neutrons atomic nucleus. Gell-Mann also coined the term "gluon ball" to describe hypothetical particles made up of several gluons.
Neutrino
These are chargeless particles that are formed during certain types of radioactive decay. They have minuscule mass even by the standards of subatomic particles. The word “neutrino” itself means “neutral particle” in Italian. Its possible existence was first announced in 1930 by Wolfgang Pauli and called it a “neutron.” However, three years later, Enrico Fermi renamed the particle in the Italian manner, “neutrino,” because by that time the term “neutron” (from the Latin “neutral,” i.e., without charge) had already begun to refer to an uncharged large particle present in any atomic nucleus.
Electron
A particle that has an indivisible amount of negative electrical charge. This name was proposed in 1894 by the Irish physicist George Johnston Stoney (1826-1911); The scientist used the ancient Greek word meaning “amber” because naturalists in ancient Greece noticed that if you rub a piece of amber with wool, it begins to attract small objects (since, as we say today, it “becomes electrified”).
Meson
This is a particle made up of one quark and one antiquark. Its name comes from the Greek word "meso", meaning "average", because mesons, when physicists first observed them and measured their characteristics, had a mass intermediate between the masses of an electron and a nucleon (a general term for the particles that form the atomic nucleus, i.e. . for protons and neutrons).
Muon
When physicists, who still did not have the same imagination as the lyricists, lacked poetic images, they named the newly discovered particles by the letters of the alphabet, albeit Greek. In this case, the letter “mu” is taken as a basis and the Greek suffix “-on”, already traditional in particle physics, is added. At first, scientists thought that this particle was a type of meson (a mu meson, as opposed to, say, a pi meson), but then they realized their mistake and accordingly renamed the mu meson muon. Mesons, as it became clear over time, are not elementary particles, since they are formed from quarks, while the muon is a full-fledged elementary particle. The muon was one of the particles that CERN scientists used to discover the Higgs boson. The researchers used a detector known as a "compact muon solenoid" to measure the energy and momentum of muons, photons, electrons and other particles produced by hadron collisions at the Large Hadron Collider.
(continued in the next issue)
Alexander Zayakin
In which there is information that all the elementary particles that make up any chemical element consist of a different number of indivisible phantom Po particles, I became interested in why the report does not talk about quarks, since it is traditionally believed that they are structural elements of elementary particles.
The theory of quarks has long become generally accepted among scientists who study the microworld of elementary particles. And although at the very beginning the introduction of the concept of “quark” was a purely theoretical assumption, the existence of which was only supposedly confirmed experimentally, today this concept is operated as an inexorable truth. The scientific world has agreed to call quarks fundamental particles, and over several decades this concept has become the central theme of theoretical and experimental research in the field of high-energy physics. “Quark” was included in the curriculum of all natural science universities in the world. Enormous funds are allocated for research in this area - just what does it cost to build the Large Hadron Collider. New generations of scientists, studying the theory of quarks, perceive it in the form in which it is presented in textbooks, with virtually no interest in the history of this issue. But let's try to unbiasedly and honestly look at the root of the “quark question”.
By the second half of the 20th century, thanks to the development of the technical capabilities of elementary particle accelerators - linear and circular cyclotrons, and then synchrotrons, scientists were able to discover many new particles. However, they did not understand what to do with these discoveries. Then the idea was put forward, based on theoretical considerations, to try to group particles in search of a certain order (similar to the periodic system of chemical elements - the periodic table). Scientists agreed name heavy and medium-mass particles hadrons, and further divide them into baryons And mesons. All hadrons participated in the strong interaction. Less heavy particles are called leptons, they participated in electromagnetic and weak interactions. Since then, physicists have tried to explain the nature of all these particles, trying to find a common model for all that describes their behavior.
In 1964, American physicists Murray Gell-Mann (Nobel Prize winner in physics 1969) and George Zweig independently proposed a new approach. A purely hypothetical assumption was put forward that all hadrons consist of three smaller particles and their corresponding antiparticles. And Gell-Man named these new particles quarks. It’s interesting that he borrowed the name itself from James Joyce’s novel “Finnegan’s Wake,” where the hero often heard words about the mysterious three quarks in his dreams. Either Gell-Man was too emotional about this novel, or he simply liked the number three, but in his scientific works he proposes to introduce the first three quarks, called the top quark, into elementary particle physics. (And - from English up), lower (d— down) and strange (s- strange), having a fractional electric charge of + 2/3, - 1/3 and - 1/3, respectively, and for antiquarks, assume that their charges are opposite in sign.
According to this model, protons and neutrons, which scientists assume make up all the nuclei of chemical elements, are composed of three quarks: uud and udd, respectively (those ubiquitous three quarks again). Why exactly out of three and in that order was not explained. It’s just something that authoritative scientific men came up with and that’s it. Attempts to make a theory beautiful do not bring us closer to the Truth, but only distort the already distorted mirror in which a piece of It is reflected. By complicating the simple, we move away from the Truth. And it's so simple!
This is how “high-precision” generally accepted official physics is built. And although the introduction of quarks was initially proposed as a working hypothesis, after a short time this abstraction became firmly established in theoretical physics. On the one hand, it made it possible from a mathematical point of view to solve the issue of ordering a vast series of open particles, on the other hand, it remained only a theory on paper. As is usually done in our consumer society, a lot of human effort and resources were directed toward experimental testing of the hypothesis of the existence of quarks. Taxpayer funds are spent, people need to be told about something, show reports, talk about their “great” discoveries in order to receive another grant. “Well, if it’s necessary, then we’ll do it,” they say in such cases. And then it happened.
A team of researchers from the Stanford Department of the Massachusetts Institute of Technology (USA) used a linear accelerator to study the nucleus, firing electrons at hydrogen and deuterium (a heavy isotope of hydrogen, the nucleus of which contains one proton and one neutron). In this case, the angle and energy of electron scattering after the collision were measured. In the case of low electron energies, the scattered protons with neutrons behaved like “homogeneous” particles, slightly deflecting the electrons. But in the case of high-energy electron beams, individual electrons lost a significant part of their initial energy, scattering at large angles. American physicists Richard Feynman (Nobel Prize winner in physics 1965 and, incidentally, one of the creators of the atomic bomb in 1943-1945 at Los Alamos) and James Bjorken interpreted electron scattering data as evidence of the composite structure of protons and neutrons, namely : in the form of previously predicted quarks.
Please pay attention to this key point. Experimenters in accelerators, colliding beams of particles (not single particles, but beams!!!), collecting statistics (!!!) saw that the proton and neutron consist of something. But from what? They didn’t see quarks, and even in the number of three, this is impossible, they just saw the distribution of energies and the scattering angles of the particle beam. And since the only theory of the structure of elementary particles at that time, albeit a very fantastic one, was the theory of quarks, this experiment was considered the first successful test of the existence of quarks.
Later, of course, other experiments and new theoretical justifications followed, but their essence is the same. Any schoolchild, having read the history of these discoveries, will understand how far-fetched everything in this area of physics is, how simply dishonest everything is.
This is how experimental research is carried out in the field of science with a beautiful name - high energy physics. Let's be honest with ourselves, today there is no clear scientific justification for the existence of quarks. These particles simply do not exist in nature. Does any specialist understand what actually happens when two beams of charged particles collide in accelerators? The fact that the so-called Standard Model, which is supposedly the most accurate and correct, was built on this quark theory does not mean anything. Experts are well aware of all the flaws of this latest theory. But for some reason it is customary to remain silent about this. But why? “And the biggest criticism of the Standard Model concerns gravity and the origin of mass. The standard model does not take gravity into account and requires that the mass, charge and some other properties of particles be measured experimentally for subsequent inclusion in equations."
Despite this, huge amounts of money are allocated to this area of research, just think about it, to confirm the Standard Model, and not to search for the Truth. The Large Hadron Collider (CERN, Switzerland) and hundreds of other accelerators around the world have been built, awards and grants are given out, a huge staff of technical specialists is maintained, but the essence of all this is a banal deception, Hollywood and nothing more. Ask any person what real benefit this research brings to society - no one will answer you, since this is a dead-end branch of science. Since 2012, there has been talk about the discovery of the Higgs boson at the accelerator at CERN. The history of these studies is a whole detective story, based on the same deception of the world community. It is interesting that this boson was allegedly discovered precisely after there was talk of stopping funding for this expensive project. And in order to show society the importance of these studies, to justify their activities, in order to receive new tranches for the construction of even more powerful complexes, CERN employees working on these studies had to make a deal with their conscience, wishful thinking.
The report “PRIMODIUM ALLATRA PHYSICS” contains the following interesting information on this subject: “Scientists have discovered a particle supposedly similar to the Higgs boson (the boson was predicted by the English physicist Peter Higgs (1929), according to the theory , it must have finite mass and no spin). In fact, what scientists discovered is not the sought-after Higgs boson. But these people, without even realizing it, made a really important discovery and discovered much more. They experimentally discovered a phenomenon that is described in detail in the AllatRa book. (note: AllatRa book, page 36, last paragraph). .
How does the microcosm of matter actually work? The report “PRIMODIUM ALLATRA PHYSICS” contains reliable information about the true structure of elementary particles, knowledge that was known to ancient civilizations, for which there is irrefutable evidence in the form of artifacts. Elementary particles consist of different numbers phantom Poe particles. “A phantom Po particle is a clot consisting of septons, around which there is a small rarefied septonic field of its own. The phantom Po particle has an internal potential (it is its carrier), which is renewed in the process of ezoosmosis. According to the internal potential, the phantom Po particle has its own proportionality. The smallest phantom Po particle is the unique power phantom particle Po - Allat (note: for more details, see later in the report). A phantom Po particle is an ordered structure in constant spiral motion. It can only exist in a bound state with other phantom Po particles, which in a conglomerate form the primary manifestations of matter. Due to its unique functions, it is a kind of phantom (ghost) for the material world. Considering that all matter consists of phantom Po particles, this gives it the characteristic of an illusory structure and a form of being dependent on the process of ezoosmosis (filling of internal potential).
Phantom Poe particles are an intangible formation. However, in concatenation (serial connection) with each other, built according to the information program in a certain quantity and order, at a certain distance from each other, they form the basis of the structure of any matter, determine its diversity and properties, thanks to their internal potential (energy and information). A phantom Po particle is what elementary particles (photon, electron, neutrino, etc.) are basically made of, as well as particles that carry interactions. This is the primary manifestation of matter in this world."

After reading this report, having conducted such a small study of the history of the development of the theory of quarks and high-energy physics in general, it became clear how little a person knows if he limits his knowledge only to the framework of a materialistic worldview. Some crazy assumptions, probability theory, conditional statistics, agreements and lack of reliable knowledge. But people sometimes spend their lives on this research. I am sure that among scientists and this field of physics there are many people who really came to science not for the sake of fame, power and money, but for the sake of one goal - knowledge of the Truth. When the knowledge of the “PRIMODIUM ALLATRA PHYSICS” becomes available to them, they themselves will restore order and make truly epoch-making scientific discoveries that will bring real benefits to society. With the publication of this unique report, a new page in world science has opened today. Now the question is not about knowledge as such, but about whether people themselves are ready for the creative use of this Knowledge. It is within the power of every person to do everything possible so that we all overcome the consumer format of thinking imposed on us and come to understand the need to create the foundations for building a spiritually creative society of the future in the coming era of global cataclysms on planet Earth.
Valery Vershigora
Keywords: quarks, quark theory, elementary particles, Higgs boson, PRIMORDIAL ALLATRA PHYSICS, Large Hadron Collider, future science, phantom Po particle, septon field, allat, knowledge of truth.
Literature:
Kokkedee Y., Theory of quarks, M., Publishing House "Mir", 340 pp., 1969, http://nuclphys.sinp.msu.ru/books/b/Kokkedee.htm;
Arthur W. Wiggins, Charles M. Wynn, The Five Biggest Unsolved Problems in Science, John Wiley & Sons, Inc., 2003 // Wiggins A., Wynn C. “Five Unsolved Problems of Science” in trans. into Russian;
Observation of an Excess of Events in the Search for the Standard Model Higgs boson with the ATLAS detector at the LHC, 09 Jul 2012, CERN LHC, ATLAS, http://cds.cern.ch/record/1460439 ;
Observation of a new boson with a mass near 125 GeV, 9 Jul 2012, CERN LHC, CMS, http://cds.cern.ch/record/1460438?ln=en ;
Report “PRIMODIUM ALLATRA PHYSICS” by an international group of scientists of the International Social Movement “ALLATRA”, ed. Anastasia Novykh, 2015;