Research methods in histology, cytology and embryology Part I
Ivanovo State Medical AcademyDepartment of Histology, Embryology and Cytology
Research methods in
histology, cytology and
embryology
Part I
Ph.D., senior lecturer M.R. Grineva
Doctor of Medical Sciences, Professor S.Yu. Vinogradov
Doctor of Medical Sciences, Professor S.V. Dindyaev
furtherIntroduction
Methods for studying living cells and tissues
Types of histological preparations of fixed cells
Making a histological specimen
Histological specimen
Taking material
Fixing the material
Material compaction
Preparation of sections
Types of microtomes
Section staining
Staining methods
Types of Dyes
Placing sections in a preservative medium
Microscopy methods
Light microscopy
Light microscope device
Microscopy technique
Dark-field microscopy
Polarization microscopy
Phase contrast microscopy
Fluorescence (luminescent) microscopy
Electron microscopy
Recommended reading
back
Further
Introduction
In modern histology, cytology and embryology they usea variety of research methods allowing for a comprehensive study
processes of development, structure and function of cells, tissues and organs.
The main stages of cytological and histological analysis are
selection of research object
preparing it for microscopy
application of microscopy methods
qualitative and quantitative image analysis
Objects
research
serve
histological
made from living or fixed cells.
drugs,
table of contents further
Methods for studying living cells and tissues
The study of living cells and tissues allows us to obtain the most completeinformation about their life activity - to trace the processes of movement,
cell division, destruction, growth, differentiation and interaction,
the duration of their cell cycle, reactive changes in response to
the action of various factors.
Methods
Lifetime
in the body (in vivo)
Implantation of transparent cameras
Intravital microscopy
Transplantation
Lifetime in culture
cells and tissues (in vitro)
Suspension cultures
Monolayer cultures
In vivo cultivation
back
table of contents further
Types of histological preparations of fixed cells
Slicethin (thickness
more than 1 micron)
semi-thin
(thickness less
1 µm)
ultra-thin
(thickness less
0.1 µm)
Smear
blood
red
bone
brain
spinal
liquids
saliva
vaginal
and etc.
Imprint
spleen
thymus
liver
mucous membrane
shell
urinary
bubble
mucous membrane
shell
cheeks
and etc.
back
Film
peritoneum
pleura
soft cerebral
shell
connecting
fabrics
and etc.
table of contents further
Making a histological specimen
backtable of contents further
Histological specimen
Histological preparations, as a rule, are sections (thick5-15 µm) organs, tissues or cells, stained with special histological
dyes.
The histological specimen must meet the following requirements:
maintain the lifetime state of structures;
be thin and transparent enough to be examined under
microscope in transmitted light;
be contrasting, that is, the structures being studied must be under
clearly defined by a microscope;
preparations for light microscopy must be preserved for a long time and
used for re-learning.
The process of manufacturing a histological preparation includes the following
main stages:
1. Taking and fixing the material
2. Material compaction
3. Preparation of sections
4. Staining of sections
5. Enclosing sections in a transparent medium
back table of contents next
Taking material
The preparation of a histological preparation is made from organs and tissuesreceived in several ways:
biopsy (puncture),
operationally,
sectional (cadaveric) material,
experimental
The following points must be taken into account:
1. Material collection should be carried out as soon as possible after death
or slaughter of an experimental animal, and if possible from a live one
object (biopsy) in order to better preserve the structure of the cell, tissue or
organ.
2. The collection of pieces must be done with a sharp instrument so as not to
injure tissue.
3. The thickness of the piece should not exceed 5mm so that the fixing
the solution could penetrate into the thickness of the piece.
4. Required
produced
marking
piece
(indicated
name of the organ, animal number or person's surname, date
fence and so on).
back table of contents next
Fixing the material
The purpose of fixing the material is to preserve the intravital morphologycells and tissues, preventing autolysis and post-mortem changes.
The fixative causes protein denaturation and lipid stabilization, thereby
thereby suspends metabolic processes and preserves structures in their
lifetime condition.
Fixation is most often achieved by immersing the piece in fixing
liquids that can be simple (formalin, alcohols, glutaric
aldehyde, acetone) and complex (Carnoy's solution, Zenker's fixative, etc.).
Fixation can also be achieved by freezing (cooling in a jet
CO2, liquid nitrogen, etc.).
The selection of fixators and duration of fixation is individual for
various organs and tissues and usually ranges from 2 to 24 hours.
back table of contents next
Material compaction
The purpose of this stage is to give the material under study suchdensity, which will allow you to obtain thin sections of the required thickness.
This is achieved in two ways:
Freezing the sample followed by freezing cutting
microtome.
Impregnation with sealing media (paraffin, epoxy resins, etc.)
The main stages of paraffin wiring:
Rinsing the material with running tap water to remove
retainer.
Dehydration (dehydration) of material in alcohols increases
concentrations (70, 80, 90, 96, absolute - 100%).
Removing alcohol and preparing the material for paraffin impregnation
treatment with paraffin solvents (xylene, etc.) and a mixture of paraffin and
xylene (at a temperature of 37°C)
Pouring into clean molten paraffin (at a temperature of 56°C).
Cooling of paraffin and formation of blocks.
back table of contents next
Preparation of sections
To produce thin sections of a given thickness at presentspecial devices are used - microtomes (for light microscopy)
and ultramicrotomes (for electron microscopy).
Special microtome knives allow you to obtain sections with the following thickness:
3-8 microns from material embedded in paraffin,
10-25 microns from material frozen in the microtome-cryostat chamber
0.08-0.1 µm from material prepared for electron microscopy
The resulting sections are placed on glass slides (for light
microscopy) or mounted on special grids (for electronic
microscopy).
back table of contents next
Types of microtomes
tobogganrotary
cryostat
freezing
for express diagnostics,
histochemistry
vibrotome
manufacturing
paraffin
slices
manufacturing
serial
paraffin
slices
manufacturing
cuts at
temperature
-20°C and below
for histochemistry
and immunocytochemistry
manufacturing
fixed and
unfixed tissues
back table of contents next
Section staining
Cellularstructures
without
special
processing,
How
as a rule,
Not
visible even at high microscope magnification. They are colorless and
transparent.
To identify tissue components, individual cells, intracellular
structures use dyes - substances with a high affinity for various
fabric components and with certain color optical properties.
The ability of tissue components to stain differently depends on
acid-base (alkaline) properties of the substances included in their composition.
Before staining, the sections are deparaffinized by sequentially
through paraffin solvent (xylene), alcohols of descending concentration (100,
96, 90, 80, 70%) and placed in water.
back table of contents next
Staining methods
General histologicalSpecial
Histochemical
identification
general plan
buildings
cells, tissues,
organs
identification
specialized
structures in
cells and
fabrics
analysis
chemical
cell composition
And
intercellular
substances
back
Impregnation
identification
specialized
structures in
cells and
fabrics
table of contents
Further
Impregnation
Method for identifying tissue structures by soaking objectshistological examination with solutions of heavy and precious salts
metals (for example, silver nitrate (silver plating), cobalt, chloride
gold (gilding), cadmium, osmium anhydride, etc.).
Areas of tissue in which metal salts are deposited on
histological structures, acquire a black or brown color in
depending on the amount and properties of the recovered metal.
Peripheral nerve
(cross section).
Oxide impregnation
osmia
Multipolar neuron.
Impregnation with silver nitrate
Multipolar neurons.
Impregnation with silver nitrate
back table of contents next
Types of general histological dyes
basicgrounds,
contacting
acidic
connections
histological
structures, cause
usually them
blue violet coloring
basophilia
metachromasia
neutral
sour
contain both
basic and
sour coloring
Components
connecting with
main
(alkaline)
connections
histological
structures,
paint them in
dye colors
neutrophilia
oxyphilia
back
table of contents further
Basophilia
Basic (alkaline) dyes actively bind to structures thatcontain acids and carry a negative charge - for example, DNA, RNA.
These include, in particular, hematoxylin, toluidine blue, thionine,
methylene blue, azure, etc.
The ability to be colored with basic (alkaline) dyes is called
basophilia (from the Greek basis - basis and philia - love).
Therefore, the structures that bind these dyes are called basophilic.
In a cell, the nucleus has basophilia (due to the high content of DNA and RNA),
sometimes the cytoplasm (with a high content of ribosomes or granular EPS).
The intercellular substance of some tissues can be stained basophilically - for example,
cartilaginous.
Nuclear basophilia
neutrophilic granulocyte.
Magnification: x630.
back
table of contents further
Metachromasia
Metachromasia (from the Greek meta - change and chroma - color, paint) - color changesome basic dyes when bound to structures that have
specific chemical properties (usually high concentration
sulfated glycosaminoglycans).
Such dyes include toluidine blue, azure II, thionine, etc.
Basophil granules have the ability to stain metachromatically.
leukocytes, mast cells.
These dyes stain other basophilic structures in the same tissues in
their usual color, i.e. orthochromatically (from the Greek orthos - correct and
chroma – paint).
Metachromasia granularity
basophilic granulocyte.
Staining according to Romanovsky-Giemsa.
Magnification: x630.
back table of contents next
Oxyphilia
Acidic dyes bind to structures that have a positive charge -for example, proteins.
Such dyes include eosin, orange G, erythrosine, picric acid, etc.
The ability to stain with acidic dyes is called oxyphilia, or
acidophilia (from Greek oxys or Latin acidus - sour and Greek philia - love).
Structures connecting
acidophilic.
these
dyes,
are called
oxyphilic
or
Oxyphilia is characteristic of the cytoplasm of cells (especially with a high content in
of mitochondria and some protein secretory granules), red blood cells (thanks to
high concentration of hemoglobin in them). Cytoplasm is oxyphilic stained
cardiomyocytes, muscle fibers of skeletal muscles, some components
intercellular substance (for example, collagen fibers).
Oxyphilia granularity
eosinophilic granulocyte.
Staining according to Romanovsky-Giemsa.
Magnification: x630.
back
table of contents further
Neutrophilia
Neutrophilia(from
lat.
neutral
–
neither
That,
neither
another,
And
philia - predisposition, love) - the ability of histological structures
can be stained with both acidic and basic dyes.
Neutrophilia granularity
neutrophilic granulocyte.
Staining according to Romanovsky-Giemsa.
Magnification: x630.
back
table of contents further
Placing sections in a preservative medium
Stained histological preparations are dehydrated in alcoholsascending concentration (70, 80, 90, 96, absolute - 100%) and
cleared in xylene, benzene, toluene or some oils.
For long-term storage, the dehydrated histological section is enclosed
(mounted) in a transparent preservative medium (resin of coniferous trees -
Canadian, fir balsam, as well as in synthetic media).
On a permanent histological specimen, the tissue section is located on
glass slide, covered with a cover glass on top. Between the glasses
(subject and cover) there is a filling medium that has
refractive index of light rays close to that of glass.
back table of contents next
Microscopy methods
table of contents furtherMicroscopy methods
OpticalLight
Polarization
Darkfield
Phase contrast
Electronic
Translucent
(transmission)
Scanning
(raster)
Fluorescent
(luminescent)
back table of contents next
Light microscopy
The histological specimen is studied in transmitted lightusing a light microscope.
Light source natural or artificial (various lamps). Light
collected in a condenser and then directed through the preparation into the lens.
The eyepiece further magnifies this image.
Quality
Images
(definition)
determined
permissive
ability of the microscope, i.e. minimum (resolving) distance,
in which the optics of the microscope make it possible to distinguish separately two close
located points. This quantity is proportional to the light wavelength and
for a conventional light microscope it is approximately 0.2 µm.
The smaller the resolving distance, the higher the resolution
microscope and even smaller objects can be examined.
Microscope magnification is the ratio between true sizes
of the object under study and the size of its image obtained using
microscope Roughly it is estimated as the product of increases
lens and eyepiece and can reach 2500 times.
back table of contents next
Light microscope device
43
5
2
6
7
8
1
12
11
10
9
Microscope base
Tube holder
Tube
Eyepiece (usually ×7)
Microscope revolver
Lenses
a) dry: ×8, ×20, ×40
b) immersion ×90
7. Stage
8. Condenser
9. Macrometric screw
10.Micrometric screw
11.Condenser screw
12.Mirror
1.
2.
3.
4.
5.
6.
Total microscope magnification = objective magnification × eyepiece magnification
back
table of contents further
Microscopy technique
1. Microscopy of a histological specimen begins with the installation of the correctlighting. To do this, using a concave mirror that collects the scattered beam
light and condenser achieve uniform illumination of the field of view.
2. The histological specimen is placed on the stage with the cover glass facing up.
3. The study of the histological specimen begins at low magnification (x8 lens), with
In this case, the distance between the objective and the cover glass should be about 1 cm.
Sharpness is adjusted using a macroscrew.
4. Examine the details of the histological specimen over the entire area, moving it to
subject table.
5. Place the area of the histological specimen in the center of the field of view, which should be
study at high magnification (x40 lens).
6. Using a revolving device, a lens with a stronger magnification is placed
(x40). The sharpness is adjusted using a microscrew.
7. To study very small histological structures, immersion is used
lens (x90).
A drop of immersion oil is applied to the cover glass of the preparation.
Carefully lower the tube until the objective lens touches the oil.
The sharpness is adjusted using a microscrew.
After completion of work, the immersion oil is removed from the lens and coverslip.
glass with gauze.
back table of contents next
Microscopy techniques (examples)
Bud.Staining: hematoxylin-eosin.
Magnification: x 56
(low magnification).
Bud.
Staining: hematoxylin-eosin.
Magnification: x 280
(high magnification).
Bud.
Staining: hematoxylin-eosin.
Magnification: x 630
back
(immersion
increase).
table of contents further
Dark-field microscopy
Based on the use of a special condenser that illuminatesthe drug with “oblique” rays that do not enter the lens.
When there is an object in the field of view, light is reflected from it and
directed towards the lens.
The method is often used to study living, unstained cells.
back
table of contents further
Polarization microscopy
Allows you to detect birefringence - anisotropy.A polarized beam of light is directed at the object of study, i.e. rays of light
directed strictly in one plane.
This is provided by a special filter - a polarizer. This light is directed towards an object
research.
The second filter - analyzer is located between the lens and the eyepiece and allows
record the angle of deviation of the plane of polarization of light.
Microscopy allows you to record the spatial arrangement of molecules in
lens or crystal structures.
Oxalate crystals.
Polarization
microscopy.
Magnification x100
back table of contents next
Phase contrast microscopy
The method is used to obtain contrast images of transparent and colorless objects, inIn particular, it allows the study of living unstained preparations.
Even with very small differences in the refractive indices of different elements of the preparation
the light wave passing through them undergoes different phase changes (acquires
phase relief). These phase changes, not perceived by the eye, are converted using
special optical device (ring diaphragm in the condenser and phase plate in
lens) in changes in the amplitude of the light wave, i.e. in changes in brightness (“amplitude
relief"), which are already visible to the eye.
In other words, in the resulting visible image the distribution of brightness (amplitude)
reproduces the phase relief. The image obtained in this way is called phase contrast.
Pseudotrichonympha grassi.
Uncolored preparation.
Phase contrast
Rat testes.
Uncolored preparation.
Phase contrast
Rat testes.
Staining: hematoxylin-eosin
Light microscopy
back table of contents next
Fluorescence (luminescence) microscopy
Uses the principle of glow of the object of study when it is illuminatedultraviolet rays. The light source is special lamps.
There is autofluorescence - intrinsic or primary
fluorescence. For example, the glow of elastic fibers in the artery wall.
Secondary fluorescence occurs after drug processing
special dyes - fluorochromes (acridine orange, rhodamine,
fluorescein, etc.).
For example: after treatment with acridine orange, the cage is very clear
nuclear DNA (bright green light) and RNA (bright red light) are detected
glow). After fixing the tissues in formaldehyde vapor (Falck method)
is discovered
bright green
glow
serotonin,
catecholamines
(adrenaline, norepinephrine).
If fluorescent dyes are coupled with specific antibodies
– it will be possible to identify their antigens. This method is called
immunocytochemical.
back table of contents next
Fluorescence (luminescent) microscopy (examples)
..
.
.
Cytoskeleton of eukaryotes
(bovine endothelial cells).
Immunocytochemical method
staining.
Actin microfilaments
painted red,
microtubules - green, nuclei
cells - blue.
Nucleic acids
in the epithelium of the uterine
iron
Acridine staining
orange.
Nuclear DNA is colored
green color,
RNA - in red.
back
Sympathetic
nerve plexuses.
Falk method
table of contents further
Electron microscopy
An electron microscope is a device that allows you to obtain images of objects withmaximum magnification up to 106 times. This became possible thanks to the use
instead of the light flux of an electron beam, the wavelength of which is many times shorter
wavelength of visible light photons.
An electron microscope consists of an electron gun (a device for obtaining
electron beam) and a system of electromagnetic lenses placed in the column
microscope under vacuum conditions.
The resolution of the electron microscope is 1000÷10000 times greater than
resolution of a light microscope and for the best modern instruments can
be less than 0.1 nm (10-10m).
Exist
two
basic
varieties
electronic
transmission (transparent) and scanning (raster).
back
microscopy:
table of contents further
Transmission electron microscopy
The operating principle of a transmission electron microscope is thatelectrons passing through an object located near the objective lens,
interact with its atoms and deviate from the original direction of incidence
beam (scatter). Then they enter a system of magnetic lenses, which
form an image of the internal
object structure. In this case, it is possible to achieve a resolution of the order of 0.1 nm, which
corresponds to magnifications up to 1.5 106 times.
The resolution and information content of TEM images are largely determined
characteristics of the object and the method of its preparation. To obtain contrast
images use ultrathin sections (no more than 0.01 µm), processed
compounds of heavy metals (impregnation with salts of lead, uranium, osmium, etc.),
selectively interacting with microstructure components (chemical
contrasting). Moreover, the greater the scattering ability of
section of the object under study (areas of increased density, increased thickness and
etc.), the darker its image will be.
back table of contents examples
Scanning (raster) electron microscopy
The operating principle of a scanning electron microscope (SEM) isscanning the sample surface with a focused electron beam and analyzing
particles reflected from it and X-ray radiation resulting from
interaction of electrons with matter.
In an SEM, a beam of electrons (electron probe) is focused by electromagnetic
condenser and objective lenses. A special device - the deflector deflects
an electron beam (primary electrons) that slides along the surface (raster).
Secondary electrons (reflected from the surface) are perceived by the detector and
are focused on the SEM screen, creating a three-dimensional image of it.
Modern SEM allows you to work in a wide range of magnifications
from approximately x10 (equivalent to the magnification of a strong hand lens)
up to x1,000,000, which is approximately 500 times the increase limit of the best
optical microscopes.
The scanning surface must be sprayed with metal: platinum, gold,
palladium, etc.
back table of contents examples
Electron microscopy (examples)
transmissionscanning
.
.
.
Red blood cells in an arteriole
.
.
.
Mast cell
back
Erythrocyte,
platelet,
leukocyte
table of contents
further1. Histology, cytology and embryology: Textbook. / Ed. Yu.A.Afanasyeva,
S.L. Kuznetsova, N.A. Yurina. – M.: Medicine, 2006. – 768 p.
2. Histology, embryology, cytology: Textbook. /
Yu.A. Chelysheva. – M.: “GEOTAR-Media”, 2007. – 408 p.
Under
ed.
E.G. Ulumbekova,
3. Junqueira L.K., Carneiro J. Histology: Atlas: Educational pos.; lane from English, ed. V.L.
Bykova. – M.: “GEOTAR-Media”, 2009. – 576 p.
4. Ham A., Cormack D. Histology: in 5 volumes; lane from English – M.: Mir, 1982.
back
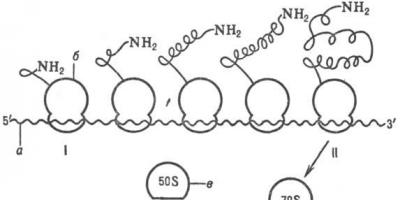
contents This method is the basic, or classic one. To make them, the test subject is immersed in fixing fluids, which denature proteins and stabilize the specific structures and compounds to be examined. The most common fixative is formalin. It cross-links proteins with methylene bridges, causing them to denature. After fixing and washing in water, the object of study can be cut into thin slices, having first frozen it on a special freezing microtome - a device used to prepare histological sections. To freeze an object, liquid carbon dioxide or an electric freezing unit is most often used. However, with this method of processing the material, rather thick histological sections are obtained. To produce thinner sections, up to 2 microns thick, the object of study must be impregnated with a substance that would make it denser. Such substances are paraffin, gelatin and celloidin. After fixing and washing, the object is sequentially immersed in alcohols of increasing concentrations from 50 to 100 degrees to dehydrate it and impregnated with gelatin, paraffin or celloidin. Once the object has been soaked and compacted, it can be cut using a microtome.
Histological sections are then stained with specially selected dyes, most of which selectively stain the structural components of cells and tissues. All dyes can be divided into three groups:
After staining, histological sections are quickly dehydrated in alcohols, cleared in xylene or toluene, transferred to a glass slide, covered with a thin layer of Canada balsam or polystyrene, and covered with a coverslip. Balsam, polystyrene and glass have the same refractive index of light, and light rays are minimally scattered when passing through the preparation.
Main Objects of research are histological preparations, and the main research method is microscopy.
The histological specimen must be sufficiently transparent (thin) and contrast. It is made from both living and dead (fixed) structures. The specimen can be a suspension of cells, a smear, an imprint, a film, a total mount, or a thin section.
The process of manufacturing histological preparations for microscopic studies includes the following main stages: 1) taking material and fixing it; 2) material compaction; 3) preparation of sections; 4) staining or contrasting sections; 5) conclusion of sections.
For staining, special histological dyes with different pH values are used: acidic, neutral and basic. The structures stained by them are, respectively, called oxyphilic, neutrophilic (heterophilic) and basophilic.
What methods does histological science use? They are quite numerous and varied:
Microscoping.
Light microscopy. Modern microscopes have high-resolution capabilities. Resolution is determined by the smallest distance (d) between two adjacent points that can be seen separately. This distance depends on the light wavelength (λ) and is expressed by the formula: d = 1/2 λ.
The minimum wavelength of the visible part of the spectrum is 0.4 microns. Consequently, the resolution of a light microscope is 0.2 μm, and the total magnification reaches 2500 times.
Ultraviolet microscopy . The wavelength of ultraviolet light is 0.2 microns, therefore, the resolution of an ultraviolet microscope is 0.1 microns, but since ultraviolet radiation is invisible, a fluorescent screen is needed to observe the object under study.
Fluorescence (luminescence) microscopy. Short-wave (invisible) radiation, absorbed by a number of substances, excites their electrons, which emit light with a longer wavelength, becoming the visible part of the spectrum. In this way, we achieve an increase in the resolution of the microscope.
Phase contrast microscopy allows you to emit unpainted objects.
Polarization microscopy used to study the architectonics of histological structures, for example, collagen fiber.
Electron microscopy makes it possible to study objects magnified tens of thousands of times.
Microphotography and microcinema . These methods make it possible to study fixed objects in photographs and living microscopic objects in motion.
Methods of qualitative and quantitative research.
Histo –and cytochemistry , including quantitative, allows for a qualitative analysis of the objects under study at the tissue, cellular and subcellular levels.
Cytospectrophotometry It makes it possible to study the quantitative content of certain biological substances in cells and tissues based on the absorption of light of a certain wavelength by the dye associated with them.
Differential centrifugation allows you to separate the contents of cells that differ in their mass.
Radiography It is based on the inclusion of a radioactive label (for example, radioactive iodine, H³-thymidine, etc.) in the metabolic process.
Morphometry allows you to measure the areas and volumes of cells, their nuclei and organelles using eyepieces and object micrometers and special grids.
Application of computers for automatic processing of digital material.
Tissue culture method is the maintenance of the viability and division of cells and tissues outside the body. For this purpose, special containers with a nutrient medium are used, in which all the necessary conditions for the life of cells are created. Using this method, it is possible to study the differentiation and functional development of cells, the patterns of their malignant degeneration and development of the tumor process, intercellular interaction, damage to cells and tissues by viruses and microorganisms, the effect of drugs on metabolic processes in cells and tissues, etc.
Intravital (vital) staining used to study the phenomena of phagocytosis and activity of macrophages, filtration capacity of renal tubules, etc.
Tissue transplantation method. This method is used to study the behavior of cells and their morphofunctional state when they are transplanted into another organism. For example, this method is used to maintain the life of animals exposed to lethal doses of radiation.
Micromanipulation. This method has been used in molecular biology, genetic engineering, as well as in cloning, when using a micromanipulator the nucleus is removed from an egg with a haploid set of chromosomes and the nucleus of a somatic cell with a diploid set of chromosomes is transplanted into it.
Objects of research are divided into:
· living (cells in a drop of blood, cells in culture, etc.);
· dead or fixed, which can be taken from either a living organism (biopsy) or from cadavers.
In any case, after taking the pieces, they are exposed to fixing solutions or freezing. Fixed objects are used for both scientific and educational purposes. Preparations prepared in a certain way and used for examination under a microscope are called histological preparations.
A histological specimen can be in the form of: (a thin stained section of an organ or tissue; a smear on glass; an imprint on glass from a fracture of an organ; a thin film preparation).
A histological preparation of any form must meet the following requirements: (preserve the vital state of the structures; be thin and transparent enough to be studied under a microscope in transmitted light; be contrasting, that is, the structures being studied must be clearly defined under the microscope; preparations for light microscopy must be preserved for a long time and used for re-learning.)
These requirements are achieved during the preparation of the drug.
Research methods:
Light microscopy-Microscopy, the main method for studying drugs, has been used in biology for more than 300 years. Ultraviolet microscopy- This is a type of light microscopy. An ultraviolet microscope uses shorter ultraviolet rays with a wavelength of about 0.2 microns. Fluorescence (luminescence) microscopy- The phenomena of fluorescence consist in the fact that atoms and molecules of a number of substances, absorbing short-wave rays, go into an excited state. Phase contrast microscopy- This method is used to obtain high-contrast images of transparent and colorless objects that are invisible with conventional microscopy methods. Electron microscopy-An electron microscope uses a stream of electrons with shorter wavelengths than a light microscope.
The main stages of cytological and histological analysis are the selection of an object of study, its preparation for examination under a microscope, the use of microscopy methods, as well as qualitative and quantitative image analysis.
Most often, a section of tissue or organ is used for study. Histological preparations can be studied without special processing. For example, a prepared blood smear, print, film or organ section can be immediately examined under a microscope. But due to the fact that the structures have weak contrast, they are poorly visible in a conventional light microscope and the use of special microscopes (phase contrast, etc.) is required. Therefore, specially processed preparations are more often used: fixed, enclosed in a solid medium and colored.
The process of manufacturing a histological preparation for light and electron microscopy includes the following main steps:
1. taking the material and fixing it,
2. material compaction,
3. preparation of sections,
4. staining or contrasting sections.
For light microscopy, one more step is required - enclosing sections in balm or other transparent media.
Fixation ensures the prevention of decomposition processes, which helps preserve the integrity of the organ structures. A small sample is either immersed in a fixative (alcohol, formaldehyde, solutions of heavy metal salts, osmic acid, special fixative mixtures) or subjected to heat treatment
The compaction of the material necessary for preparing sections is carried out by impregnating the previously dehydrated material with paraffin, celloidin, and organic resins. Faster compaction is achieved by using the method of freezing the pieces, for example, in liquid carbon dioxide.
The sections are prepared using special devices - microtomes(for light microscopy) and ultramicrotomes(for electron microscopy).
Staining sections (in light microscopy) or sputtering them with metal salts (in electron microscopy) is used to increase the contrast of the image of individual structures when viewing them under a microscope. Methods for staining histological structures are very diverse and are selected depending on the objectives of the study.
Histological dyes (according to their chemical nature) are divided into acidic, basic and neutral. Common dye hematoxylin, which stains cell nuclei purple, and acidic dye - eosin, staining the cytoplasm pink-yellow. The selective affinity of structures for certain dyes is determined by their chemical composition and physical properties. Structures that stain well with acidic dyes are called oxyphilic, and those that stain with basic dyes are called basophilic. For example, the cytoplasm of cells is most often stained oxyphilic, and the nuclei of cells are stained basophilic.
Structures that accept both acidic and basic dyes are neutrophilic (heterophilic). Colored preparations are usually dehydrated in alcohols of increasing strength and cleared in xylene, benzene, toluene or some oils. For long-term preservation, the dehydrated histological section is enclosed between a slide and cover glass in Canada balsam or other substances. The finished histological specimen can be used for study under a microscope for many years.
4) . The cell as a structural and functional unit of tissue. Definition. General plan of the structure of eukaryotic cells. Biological cell membranes, their structure, chemical composition and main functions.
A cell is an elementary structural, functional and genetic unit within all plant and animal organisms. Structure of a eukaryotic cell:
The cells that form the tissues of animals and plants vary significantly in shape, size and internal structure. Cells of all types contain two main components that are closely related to each other - the cytoplasm and the nucleus. The nucleus is separated from the cytoplasm by a porous membrane and contains nuclear sap, chromatin and the nucleolus. Semi-liquid cytoplasm fills the entire cell and is penetrated by numerous tubules. On the outside it is covered with a cytoplasmic membrane.
The cell body itself and its contents are separated from the external environment or from neighboring elements in multicellular organisms by a plasma membrane. Outside the plasma membrane is the cell membrane or wall, which is especially pronounced in plants. All the internal contents of the cell, with the exception of the nucleus, are called cytoplasm. The cytoplasm of eukaryotic cells is not homogeneous in its structure and composition and includes: hyaloplasm, membrane and non-membrane components. Membrane organelles come in two varieties: single-membrane and double-membrane. The first include organelles of the vacuolar system - the endoplasmic reticulum, Golgi apparatus, lysosomes, peroxisomes and other specialized vacuoles, as well as the plasma membrane. Double-membrane organelles include mitochondria and plastids, as well as the cell nucleus. Non-membrane organelles include ribosomes, the cellular center of animal cells, as well as cytoskeletal elements (microtubules and microfilaments).
The term hyaloplasm, the main plasma or matrix of the cytoplasm, refers to a very important part of the cell, its true internal environment. Hyaloplasm is a complex colloidal system that includes various biopolymers: proteins, nucleic acids, polysaccharides, etc. Enzymes involved in the synthesis of amino acids, nucleotides, fatty acids, and sugar metabolism are localized in it. The most important role of hyaloplasm is that this environment unites all cellular structures and ensures their chemical interaction with each other. Most of the intracellular transport processes are carried out through the hyaloplasm: the transfer of amino acids, fatty acids, nucleotides, and sugars. In the hyaloplasm there is a constant flow of ions to and from the plasma membrane, to the mitochondria, nucleus and vacuoles. In the hyaloplasm, storage products are deposited: glycogen, fat. In the cytosol, on the ribosomes located there, proteins are synthesized that are transported to different parts of the cell, as well as all the proteins of the cell nucleus, most of the proteins of mitochondria and plastids, and the main proteins of peroxisomes. Structure of cell membranes.
A common feature of all cell membranes (plasma, intracellular and membrane organelles) is that they are thin (6-10 nm) layers of lipoprotein nature (lipids in complex with proteins), closed on themselves
There are three important principles of membrane structure:
The membranes are not homogeneous. The membranes surrounding intracellular organelles and the plasma membrane differ in composition. Many membrane components are in a state of continuous movement. The membrane resembles an ever-changing mosaic. The components of the membranes are extremely asymmetrical. There is a difference in the relative quantity and qualitative composition of lipids between the outer and inner layers of membranes. Proteins are located asymmetrically among lipids and have clearly distinguishable extra- and intracellular regions.
The most important functions of membranes are the following:
Membranes control the composition of the intracellular environment.
Membranes provide and facilitate intercellular and intracellular transmission of information.
Membranes provide tissue formation through intercellular contacts
Chemical composition of the cell.
The cells of living organisms are similar not only in their structure, but also in their chemical composition. The similarity in the structure and chemical composition of cells indicates the unity of their origin.
Based on their composition, the substances entering the cell are divided into organic and inorganic.
1.Inorganic substances.
Water is of great importance in the life of a cell. Many elements in cells are contained in the form of ions. The most common cations are: K+, Na+, Ca2+ Mg2+, and anions: H2PO4-, Cl-, HCO3-.
Mineral salts (for example, calcium phosphate) can be part of the intercellular substance and shells of mollusks and provide the strength of these formations.
2. Organic substances.
Characteristic only of living things. Organic compounds are represented in the cell by simple small molecules (amino acids, mono- and oligosaccharides, fatty acids, nitrogenous bases), and macromolecules of biopolymers (proteins, lipids, polysaccharides, nucleic acids). Biopolymer molecules consist of repeating low molecular weight compounds (monomers
Today it is no longer possible to imagine the full treatment of a malignant tumor without histological examination. However, few people have a clear idea of this diagnostic method. In this article we will talk about how histological examination is carried out and why it is necessary. We will also talk about the use of diagnostics of this kind in gynecology.
What is
The histological research method consists of studying the internal tissues of the patient’s body, which are taken in the form of a small sample. Often the material is obtained through a biopsy. In diagnosing cancer tumors and assessing the correctness of drug therapy, histological examination is one of the most important stages.
Objectives
Such diagnostics are carried out to clarify or confirm a previously made diagnosis. It also helps to correctly identify the disease in controversial situations. Histological examination of the patient’s material makes it possible to identify the presence of a malignant tumor at an early stage and study the dynamics of its growth (determine whether there is growth, enlargement, or spread of the tumor). In addition, it is used to carry out differential diagnosis of pathological processes and analyze changes occurring in tissues during treatment.
Meaning in medicine
Currently, before chemotherapy or radiation treatment of patients with malignant tumors, a histological examination of the tumor is mandatory. Also, not a single oncology-related surgical operation is performed without it. In addition, a thorough examination of patient tissue is necessary in order to detect even the slightest changes in the tumor process in a timely manner and take timely measures.
But histological examination is used in the treatment of not only cancer patients. A biopsy is extremely important when choosing the optimal treatment program for patients with diseases studied by such branches and branches of medicine as gynecology, gastroenterology, urology, pulmonology, otorhinolaryngology, hematology, nephrology, thoracic and abdominal surgery, etc.
Taking material

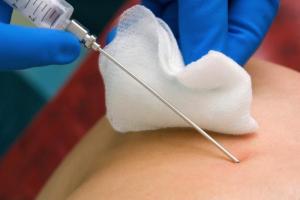
The material necessary for the study can be obtained from any tissue and internal organs of the patient. Today there are many ways to perform this procedure:
- Excision of the required amount of tissue during surgery (excisional biopsy).
- Puncture of the cavity of the affected organ or malignant tumor formation using a special long needle. These needles are available in various designs and types. For example, a histological examination of the liver is performed through a puncture biopsy.
- Cutting or cutting small pieces of tissue from removed internal organs.
- Biting off the required amount of tissue with special forceps when performing endoscopic manipulations: colonoscopy, bronchoscopy, esophagogastroduodenoscopy. This method is called a forceps biopsy.
- Suctioning out small amounts of material from hollow internal organs (aspiration biopsy).
- Curettage of the internal walls of pathological and natural cavities. In this way, for example, a histological examination of the cervix and osteomyelitic cavity is carried out.
Features of the procedure
To obtain the most reliable and correct results, it is necessary to strictly follow the rules for collecting biological material. Tissue samples, as already mentioned, can be taken during a surgical operation, when, for example, part or all of an organ is removed, or as a result of a biopsy. Most doctors prefer the second method of collecting material; it is much more common.
Histological examination can be carried out by studying both the entire tumor formation and a small column of tissue. Often a biopsy is performed using a very long and thin needle, which is intended for intramuscular injection. But in some cases, a larger diameter needle is used - this makes the procedure more painful, but also more effective, since specialists also have the opportunity to conduct immunohistochemical analysis.

Diagnostic methods
There are two methods of performing histological examination - traditional and accelerated. In the first case, the resulting tissue samples are first poured with molten paraffin, and then cut into plates 1-8 microns thick and subjected to dyeing. When using this method, the analysis data will be ready in five to ten days.
When using an accelerated technique, the result of a histological examination can be obtained within an hour. In this case, the biological material taken from the patient is urgently frozen, and then layer-by-layer thin cuts are made in it and carefully examined under a microscope. This method is indispensable when the surgeon, when performing an operation, urgently needs to make a decision regarding whether to remove or preserve the patient’s organ.
If the histological examination is planned to be performed not in the near future, but later, then the tissues are filled with alcohol, formaldehyde solution or osmic acid in order to preserve the structure. As for hard materials, they are carefully softened.
Analysis results
The histological research method is highly accurate. This is due to the fact that the tissues of the affected organ are studied under a microscope, and are not viewed through other tissues and organs, as happens during an X-ray or ultrasound examination. It is for this reason that histological analysis is considered the most important for making a final diagnosis. In addition, thanks to microscopy and mandatory staining of patient tissue, specialists have the opportunity to obtain the most accurate data on the current condition of the affected organ. Knowing the approved standards for the structure of tissues and internal organs in a healthy state, the doctor can easily assess pathological changes and quickly determine the presence of the disease, as well as its degree.

Based on the results of the study, the specialist gives a conclusion. It may contain an indicative or final diagnosis, and in some cases only a descriptive answer is given, allowing one to make only an assumption about the nature of the pathology (if there is insufficient clinical information or material).
An indicative diagnosis makes it possible to determine the range of diseases for differential research, and the final answer serves as the basis for formulating a clinical diagnosis.
Erroneous data
Many patients are interested in the question of whether erroneous results can be obtained during histological examination. This usually happens if the doctor collected the biomaterial incorrectly. For example, he took a lot of healthy tissue, but almost completely missed the affected area of the organ. Also, the cause of the error may be incorrect storage conditions for the patient’s tissues or serious violations committed during their preparation for storage.
In addition, to obtain a reliable result, the number of sections is of great importance - the more there are, the better, because if there are not enough sections, the affected area of tissue can be skipped, in which case a thorough study will not be carried out.

Often, errors in such diagnostics are explained by the insufficient qualifications of the histologist and the lack of mutual understanding between him and the doctor treating the patient.
Histological studies in gynecology
In this branch of medicine, the diagnostic method in question is of great importance. All histopathological types of examination have found their application in gynecology. Their use makes it possible to establish a diagnosis with the highest degree of reliability in the case of various pathologies of the female reproductive system. A particularly important role is played by histological examination of the uterus, its appendages, and the cervix. Such diagnostics make it possible to identify oncological diseases, as well as determine the causes of missed pregnancies and spontaneous miscarriages.
Histological examination of the endometrium
This diagnostic option in gynecology makes it possible to correctly assess the functioning of the ovaries and promptly identify any pathological processes occurring in them. If a woman is still undergoing her menstrual cycle, a histological examination of the endometrium is carried out approximately three days before the start of her next period. If the patient has dysfunctional bleeding, scrapings must be taken directly during bleeding.
Biological tissues obtained through diagnostic curettage are stained with hematoxylin or eosin for research. In some cases, the Van Gieson technique is used. After staining, the structure of the stroma and glands is determined by analysis, and all the features of the endometrium are revealed. During the luteal phase of the menstrual cycle, healthy glands have a saw-tooth shape and are slightly dilated. The cells of the glandular epithelium themselves have light cytoplasm and pale nuclei, and a secretion is necessarily found in the glands.

Histology of the cervix
Diagnosis is carried out by collecting a small amount of tissue from the lower segment of the reproductive organ. If, during the analysis, minor pathological changes are detected in them, then it can be stated that the patient has a benign formation or an inflammatory disease. If many cells with pathological changes are detected, they speak of the development of a malignant tumor or a precancerous condition.
Histology of the uterus
Histological examination of the reproductive organ is carried out exclusively according to indications. Such a diagnosis is performed if, for example, a woman suffers from pain in the lower abdomen or has prolonged uterine bleeding, if a tumor formation is detected when palpating the abdomen, and so on.
Biological material is taken for examination during diagnostic hysteroscopy - this is a minimally invasive examination of the inner surface of the reproductive organ using a hysteroscope - a special optical device. It should be noted that the procedure is quite complex and is often performed under general anesthesia (in rare cases, local anesthesia is used). Using the instruments included in the hysteroscope, the specialist takes pieces of tissue, which are then sent for histological examination, during which it is possible to determine exactly what caused the unpleasant symptoms. This diagnosis also makes it possible to distinguish benign formations (for example, fibroids) from malignant ones.

Ovarian histology
In this case, the collection of biological material for histological analysis is performed through a puncture biopsy (a puncture is made in the anterior abdominal wall). Currently, this procedure is carried out under ultrasound control - this makes it possible to obtain tissue directly from those areas that are suspicious. Carrying out such a diagnosis makes it possible to distinguish a benign tumor and cyst from ovarian cancer.