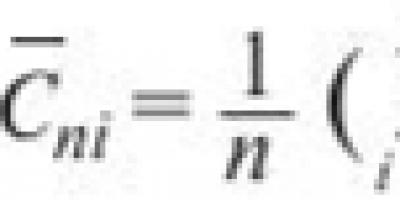These hazardous compounds are among the most important priority air (water and soil) pollutants. They enter the atmospheric air during various combustion processes and with vehicle exhaust gases (anthracene and benzo[a]pyrene) were contained in more than three-quarters of the houses surveyed.
The dangers PAHs pose to the environment
On the environmental hazard scale of 0 to 3, shown above in Figure 3, polycyclic aromatic hydrocarbons are rated 1.5. Level 3 poses a very high environmental hazard and Level 0 poses a low hazard. Factors taken into account include assessing the degree of toxicity or non-toxicity of a substance, measuring its ability to remain active in the environment and its ability to accumulate in living organisms. The release of the substance is not taken into account. This is reflected in the NPI level for a given substance. One of the substances whose environmental hazard is assessed as high is nitrogen oxide (3) and one of the substances whose hazard is assessed as low is carbon monoxide (0.8).
Toxicity of PAHs to humans
The toxicity of PAHs is highly dependent on the structure; even isomers can be either non-toxic or extremely toxic. Thus, highly carcinogenic PAHs can be small (less than 3 rings) or large (more than 4 rings). One PAH, benzo[a]pyrene, is the first carcinogen studied and is one of many carcinogens found in cigarettes. Seven PAHs have been classified as probable human carcinogens: benzo[a]anthracene, benzo[a]pyrene, benzo[ b ]fluoranthene, benzo[k]fluoranthene, chrysene, dibenz[a, h ]anthracene and indenopyrene.
PAHs known for their carcinogenic, mutagenic and teratogenic properties: benzo[a]anthracene and crisene, benzo[ b ]fluoranthene, benzo[ j ]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, benzo[ ghi ]pyrylene, coronene, dibenz[ a , h ]anthracene, indenopyrene and ovalene (Fetzer, D. K. (2000), Lach, A (2005)).
Due to the lack of representative mixtures of PAHs for research purposes, the effects of biological and non-biological modifiers on PAH toxicity and metabolism are not yet well understood.
The following safety criteria for total PAHs, carcinogenic PAHs and benzo(a)pyrene in drinking water and air and total PAHs and benzo(a)pyrene in food have been proposed: 0.01 to<0,2 мкг общих ПАУ/л, <0,002 мкг канцерогенных ПАУ/л и 0,0006 мкг бензо(а)пирена /л; воздух: < 0,01 мкг общих ПАУ/м 3 , <0,002 мкг канцерогенных ПАУ/м 3 и 0,0005 мкг бензо(а)пирена/м 3 ; пища: 1,6 до < 16,0 мкг общих ПАУ ежедневно и 0,16 до < 1,6 мкг бензо(а)пирена ежедневно.
Directions for use
Many PAHs are not used at all. But some are used in medicine, to make paints, plastics and pesticides. Mothballs, also known as mothballs, are used in the production of dyes, explosives, plastics, lubricants and moth repellents. Anthracene is used in paints, insecticides and wood preservatives.
Conclusion
From the above review it is obvious that, despite some usefulness of PAHs, their environmental and toxicological hazards are a matter of acute concern and their concentrations should be greatly reduced in the environment, and in the best case, they should be completely eliminated from it.
Literature
1. Battersby S (2004). Organic origin of cosmic molecules. January 2004, http ://www. news scientist com/news/news. jsp? id=ns99994552.
2. Cook M and A. D. Dennis. 1981. Chemical analysis and biological role: polynuclear aromatic hydrocarbons. Fifth international symposium. Battelle Press, Columbus, Ohio. 770 pp.
3. Edwards N.T. 1983. Polycyclic aromatic hydrocarbons (PAHs) in the terrestrial environment—a review. Journal of Environmental Quality 12.427-441.
4. Isman, G. A., Davani, B., and Dodson, D. A. 1984. Hydrostatic testing of gas pipelines as a source of PAHs in the aquatic environment. International Journal of Environmental Chemical Analysis. 19:27-39.
5. Isler R (1987) Effects of polycyclic aromatic hydrocarbons on fish, wildlife and invertebrates: A synoptic review.
6. U.S. Fish and Wildlife Service, Patuxent Wildlife Research Center. Laurel. EPA. 1980. Water quality in terms of polycyclic aromatic hydrocarbons. US Environmental Protection Agency. 440/5-80-069.193.
7. Fetzer DK (2000) Chemistry and analysis of heavy polycyclic aromatic hydrocarbons. NY. Willey.
8. Lee SD, Grant L. 1981. Health and environmental assessment of polycyclic aromatic hydrocarbons. Publishing house Patotex. Forest Souse Park, Illinois. 364 pp.
9. Lach A. (2005). Carcinogenic effect of polycyclic aromatic hydrocarbons. London: Imperial College Press, ISBN 1-86094-417-5.