Absolutely any chemical consists of a certain set of protons and neutrons. They are held together by the fact that the binding energy of the atomic nucleus is present inside the particle.
A characteristic feature of the nuclear forces of attraction is their very high power at relatively small distances (from about 10 -13 cm). As the distance between the particles increases, the forces of attraction inside the atom also weaken.
Reasoning about the binding energy inside the nucleus
If you imagine that there is a way to separate protons and neutrons in turn from the nucleus of an atom and arrange them at such a distance that the binding energy of the atomic nucleus ceases to act, then this must be very hard work. In order to extract its components from the nucleus of an atom, one must try to overcome the intra-atomic forces. These efforts will go towards dividing the atom into the nucleons it contains. Therefore, it can be judged that the energy of the atomic nucleus is less than the energy of the particles of which it consists.

Is the mass of subatomic particles equal to the mass of an atom?
Already in 1919, researchers learned how to measure the mass of an atomic nucleus. Most often, it is “weighed” using special technical devices, which are called mass spectrometers. The principle of operation of such devices is that the characteristics of the movement of particles with different masses are compared. Moreover, such particles have the same electric charges. Calculations show that those particles that have different mass indices move along different trajectories.
Modern scientists have found out with great accuracy the masses of all nuclei, as well as the protons and neutrons that make up them. If we compare the mass of a certain nucleus with the sum of the masses of the particles contained in it, then it turns out that in each case the mass of the nucleus will be greater than the mass of individual protons and neutrons. This difference will be approximately 1% for any chemical. Therefore, we can conclude that the binding energy of an atomic nucleus is 1% of its rest energy.

Properties of intranuclear forces
Neutrons that are inside the nucleus are repelled from each other by Coulomb forces. However, the atom does not fall apart. This is facilitated by the presence of an attractive force between particles in an atom. Such forces, which are of a nature other than electrical, are called nuclear. And the interaction of neutrons and protons is called the strong interaction.
Briefly, the properties of nuclear forces are as follows:
- this is charge independence;
- action only at short distances;
- as well as saturation, which refers to the retention of only a certain number of nucleons near each other.
According to the law of conservation of energy, at the moment when nuclear particles are combined, energy is released in the form of radiation.

Binding energy of atomic nuclei: formula
For the above calculations, the generally accepted formula is used:
E St=(Z m p +(A-Z) m n -MI) s²
Here under E St refers to the binding energy of the nucleus; With- the speed of light; Z-number of protons; (A-Z) is the number of neutrons; m p denotes the mass of the proton; A m n is the mass of the neutron. M i denotes the mass of the nucleus of an atom.
Internal energy of nuclei of various substances
To determine the binding energy of the nucleus, the same formula is used. The binding energy calculated by the formula, as previously indicated, is no more than 1% of the total atomic energy or rest energy. However, on closer examination, it turns out that this number fluctuates quite strongly from substance to substance. If you try to determine its exact values, then they will differ especially for the so-called light nuclei.
For example, the binding energy inside a hydrogen atom is zero because there is only one proton in it. The binding energy of a helium nucleus would be 0.74%. For nuclei of a substance called tritium, this number will be 0.27%. Oxygen has 0.85%. In nuclei, where there are about sixty nucleons, the intra-atomic bond energy will be about 0.92%. For atomic nuclei with a larger mass, this number will gradually decrease to 0.78%.
To determine the binding energy of the nucleus of helium, tritium, oxygen, or any other substance, the same formula is used.

Types of protons and neutrons
The main reasons for such differences can be explained. Scientists have found that all the nucleons that are contained inside the nucleus are divided into two categories: surface and internal. Internal nucleons are those that are surrounded by other protons and neutrons from all sides. Surface ones are surrounded by them only from the inside.
The binding energy of an atomic nucleus is a force that is more pronounced for internal nucleons. Something similar, by the way, occurs with the surface tension of various liquids.
How many nucleons fit in a nucleus
It has been found that the number of internal nucleons is especially small in the so-called light nuclei. And in those that belong to the category of the lightest, almost all nucleons are regarded as surface. It is believed that the binding energy of the atomic nucleus is a quantity that should increase with the number of protons and neutrons. But even this growth cannot continue indefinitely. With a certain number of nucleons - and this is from 50 to 60 - another force comes into play - their electrical repulsion. It occurs even regardless of the presence of binding energy within the nucleus.
The binding energy of the atomic nucleus in various substances is used by scientists to release nuclear energy.
Many scientists have always been interested in the question: where does the energy come from when lighter nuclei merge into heavy ones? In fact, this situation is analogous to atomic fission. In the process of fusion of light nuclei, just as occurs during the splitting of heavy ones, nuclei of a stronger type are always formed. In order to "get" all the nucleons in them from light nuclei, it is required to spend less energy than what is released when they combine. The converse is also true. In fact, the energy of fusion, which falls on a certain unit of mass, may be greater than the specific energy of fission.

Scientists who studied the processes of nuclear fission
The process was discovered by the scientists Hahn and Strassmann in 1938. Within the walls of Berlin chemical university researchers discovered that when uranium is bombarded with other neutrons, it turns into lighter elements, standing in the middle of the periodic table.
A significant contribution to the development of this field of knowledge was also made by Lise Meitner, whom Hahn once proposed to study radioactivity together. Hahn allowed Meitner to work only on the condition that she would conduct her research in the basement and never go up to the upper floors, which was a fact of discrimination. However, this did not prevent her from achieving significant success in the study of the atomic nucleus.
Themes USE codifier: binding energy of nucleons in the nucleus, nuclear forces.
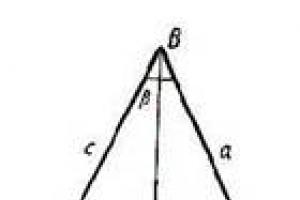
The atomic nucleus, according to the nucleon model, consists of nucleons - protons and neutrons. But what forces keep nucleons inside the nucleus?
What, for example, keeps two protons and two neutrons together inside the nucleus of a helium atom? After all, protons, repelled from each other by electric forces, would have to scatter in different directions! Perhaps this gravitational attraction of nucleons to each other does not allow the nucleus to decay?
Let's check. Let two protons be at some distance from each other. Let's find the ratio of the force of their electrical repulsion to the force of their gravitational attraction:
Proton charge C, proton mass kg, so we have:
What a monstrous superiority of electrical power! The gravitational attraction of protons not only does not ensure the stability of the nucleus - it is generally not noticeable against the background of their mutual electrical repulsion.
Consequently, there are other forces of attraction that hold the nucleons together inside the nucleus and exceed in magnitude the electrical repulsion of protons. These are the so-called nuclear forces.
Nuclear forces.
So far we have known two types of interactions in nature - gravitational and electromagnetic. Nuclear forces serve as a manifestation of a new, third type of interaction - the strong interaction. We will not go into the mechanism of the emergence of nuclear forces, but only list their most important properties.
1. Nuclear forces act between any two nucleons: proton and proton, proton and neutron, neutron and neutron.
2. The nuclear forces of attraction of protons inside the nucleus are approximately 100 times greater than the electrical repulsion of protons. More powerful forces than nuclear forces are not observed in nature.
3. Nuclear attractive forces are short-range: their radius of action is about m. This is the size of the nucleus - it is at this distance from each other that nucleons are kept by nuclear forces. As the distance increases, the nuclear forces decrease very rapidly; if the distance between the nucleons becomes equal to m, the nuclear forces will almost completely disappear.
At distances smaller than m, the nuclear forces become repulsive forces.
The strong interaction is one of the fundamental ones - it cannot be explained on the basis of some other types of interactions. The ability for strong interactions turned out to be inherent not only to protons and neutrons, but also to some other elementary particles; all such particles are called hadrons. Electrons and photons do not belong to hadrons - they do not participate in strong interactions.
Atomic mass unit.
Masses of atoms and elementary particles extremely small, and it is inconvenient to measure them in kilograms. Therefore, in the atomic nuclear physics often a much smaller unit is used - so
the called atomic unit of mass (abbreviated a. e. m.).
By definition, an atomic mass unit is 1/12 the mass of a carbon atom. Here is its value with an accuracy of five decimal places in standard notation:
A.e.m.kg
(We will later need such accuracy to calculate one very important quantity, which is constantly used in calculations of the energy of nuclei and nuclear reactions.)
It turns out that 1 a. e.m., expressed in grams, is numerically equal to the reciprocal of the Avogadro constant mole:
Why is it so? Recall that Avogadro's number is the number of atoms in 12g of carbon. In addition, the mass of a carbon atom is 12 amu. e. m. From here we have:
therefore a. e. m. = r, which was required.
As you remember, any body of mass m has a rest energy E, which is expressed by Einstein's formula:
. (1)
Find out how much energy is contained in one atomic mass unit. We will need to carry out calculations with a sufficiently high accuracy, so we take the speed of light with five decimal places:
So for mass a. e. m. we have the corresponding rest energy:
J. (2)
In the case of small particles, it is inconvenient to use joules - for the same reason as kilograms. There is a much smaller unit of energy - electron-volt(abbreviated eV).
By definition, 1 eV is the energy acquired by an electron when passing through an accelerating potential difference of 1 volt:
EV ClV J. (3)
(you remember that in problems it is enough to use the value of the elementary charge in the form of C, but here we need more accurate calculations).
And now, finally, we are ready to calculate the very important value promised above - the energy equivalent of an atomic mass unit, expressed in MeV. From (2) and (3) we get:
EV . (4)
So let's remember: rest energy of one a. e.m. is equal to 931.5 MeV. You will encounter this fact many times when solving problems.
In what follows, we will need the masses and rest energies of the proton, neutron, and electron. We present them with an accuracy sufficient for solving problems.
A. e. m., MeV;
A. e.m., MeV;
A. e. m., MeV.
Mass defect and binding energy.
We are used to the fact that the mass of a body is equal to the sum of the masses of the parts of which it consists. In nuclear physics, this simple idea has to be weaned.
Let's start with an example and take the well-known to us -particle core. In the table (for example, in Rymkevich's problem book) there is a value for the mass of a neutral helium atom: it is 4.00260 AU. e. m. To find the mass M of the helium nucleus, it is necessary to subtract the mass of two electrons in the atom from the mass of a neutral atom:
At the same time, the total mass of two protons and two neutrons that make up the helium nucleus is:
We see that the sum of the masses of the nucleons that make up the nucleus exceeds the mass of the nucleus by
The value is called mass defect. By virtue of Einstein's formula (1), a mass defect corresponds to a change in energy:
The quantity is also denoted and is called the binding energy of the nucleus. Thus, the binding energy of the -particle is approximately 28 MeV.
What is physical meaning the binding energy (and, therefore, the mass defect)?
To split a nucleus into its constituent protons and neutrons, do the job against the action of nuclear forces. This work is not less than a certain value; the minimum work on the destruction of the nucleus is performed in the case when the released protons and neutrons rest.
Well, if work is done on the system, then the energy of the system increases by the amount of work done. Therefore, the total rest energy of the nucleons that make up the nucleus and taken separately turns out to be more the rest energy of the nucleus by .
Consequently, the total mass of nucleons that make up the nucleus will also be greater than the mass of the nucleus itself. This is why a mass defect occurs.
In our example with the -particle, the total rest energy of two protons and two neutrons is 28 MeV greater than the rest energy of the helium nucleus. This means that in order to split the nucleus into its constituent nucleons, work must be done equal to at least 28 MeV. We called this quantity the binding energy of the nucleus.
So, core binding energy is the minimum work that must be done to split the nucleus into its constituent nucleons.
The binding energy of the nucleus is the difference between the rest energies of the nucleons of the nucleus, taken separately, and the rest energy of the nucleus itself. If the mass nucleus consists of protons and neutrons, then for the binding energy we have:
The quantity , as we already know, is called the mass defect.
Specific binding energy.
An important characteristic of the core strength is its specific binding energy, equal to the ratio of the binding energy to the number of nucleons:
The specific binding energy is the binding energy per nucleon, and has the meaning of the average work that must be done to remove the nucleon from the nucleus.
On fig. 1 shows the dependence of the specific binding energy of natural (that is, naturally occurring 1 ) isotopes chemical elements from the mass number A.

Rice. 1. Specific binding energy of natural isotopes
Elements with mass numbers 210–231, 233, 236, 237 do not occur naturally. This explains the gaps at the end of the graph.
For light elements, the specific binding energy increases with increasing , reaching a maximum value of 8.8 MeV / nucleon in the vicinity of iron (that is, in the range of about 50 to 65). Then it gradually decreases to a value of 7.6 MeV/nucleon for uranium.
This character of the dependence of the specific binding energy on the number of nucleons is explained by the combined action of two oppositely directed factors.
The first factor is surface effects. If there are few nucleons in the nucleus, then a significant part of them is on a surface kernels. These surface nucleons are surrounded by a smaller number of neighbors than the inner nucleons and, accordingly, interact with a smaller number of neighboring nucleons. With an increase, the proportion of internal nucleons increases, and the proportion of surface nucleons decreases; therefore, the work that needs to be done to remove one nucleon from the nucleus should, on average, increase with increasing .
However, with an increase in the number of nucleons, the second factor begins to appear - Coulomb repulsion of protons. After all, the more protons in the nucleus, the greater electrical forces repulsions tend to break the core; in other words, the stronger each proton is repelled from the other protons. Therefore, the work required to remove a nucleon from the nucleus should, on average, decrease with increasing .
As long as there are few nucleons, the first factor dominates over the second, and therefore the specific binding energy increases.
In the vicinity of iron, the effects of both factors are compared with each other, as a result of which the specific binding energy reaches a maximum. This is the region of the most stable, durable nuclei.
Then the second factor begins to outweigh, and under the influence of ever-increasing forces of Coulomb repulsion, bursting the nucleus, the specific binding energy decreases.
Saturation of nuclear forces.
The fact that the second factor dominates in heavy nuclei indicates one interesting feature nuclear forces: they have the property of saturation. This means that each nucleon in a large nucleus is connected by nuclear forces not with all other nucleons, but only with not a large number their neighbors, and this number does not depend on the size of the nucleus.
Indeed, if there were no such saturation, the specific binding energy would continue to increase with increasing - after all, then each nucleon would be held together by nuclear forces with an increasing number of nucleons of the nucleus, so that the first factor would invariably dominate over the second. The Coulomb repulsive forces would have no chance of turning the tide in their favor!
We list the main characteristics of nuclei, which will be discussed further:
- Binding energy and nuclear masses.
- Kernel sizes.
- Nuclear spin and angular momentum of the nucleons that make up the nucleus.
- Parity of the nucleus and particles.
- Isospin of the nucleus and nucleons.
- Spectra of nuclei. Characteristics of the ground and excited states.
- Electromagnetic properties of the nucleus and nucleons.
1. Binding energies and nuclear masses
The mass of stable nuclei is less than the sum of the masses of the nucleons entering the nucleus, the difference between these quantities determines the binding energy of the nucleus:
| (1.7) |
The coefficients in (1.7) are chosen from the conditions of the best fit between the model distribution curve and the experimental data. Since such a procedure can be carried out in different ways, there are several sets of Weizsäcker formula coefficients. The following are often used in (1.7):
a 1 = 15.6 MeV, a 2 = 17.2 MeV, a 3 = 0.72 MeV, a 4 = 23.6 MeV,

It is easy to estimate the charge number Z at which nuclei become unstable with respect to spontaneous decay.
Spontaneous nuclear decay occurs when the Coulomb repulsion of the protons of the nucleus begins to dominate over the nuclear forces contracting the nucleus. An estimate of the nuclear parameters at which such a situation occurs can be carried out by considering changes in the surface and Coulomb energies during deformation of the nucleus. If the deformation leads to a more favorable energy state, the nucleus will spontaneously deform until it divides into two fragments. Quantitatively, such an assessment can be carried out as follows.
During deformation, the core, without changing its volume, turns into an ellipsoid with axes (see Fig. 1.2 )
:
Thus, the deformation changes the total energy of the nucleus by the amount
It should be emphasized that the approximate nature of the result obtained as a consequence of the classical approach to a quantum system - the nucleus.
Separation energies of nucleons and clusters from the nucleus
The energy of neutron separation from the nucleus is equal to
E separate n \u003d M (A - 1, Z) + m n - M (A, Z) \u003d Δ (A - 1, Z) + Δ n - Δ (A, Z).
Proton separation energy
E separate p = M(A–1,Z–1) + M(1 H) – M(A, Z) = Δ (A–1, Z–1) + Δ (1 H) – Δ (A, Z).
It should be noted that since the main data on the masses of nuclei are tables of "excess" masses Δ, it is more convenient to calculate the separation energies using these quantities.
E separate n (12 C) = Δ (11 C) + Δ n – Δ (12 C) = 10.65 MeV + 8.07 MeV – 0 = 18.72 MeV.
15. Examples of problem solving
1. Calculate the mass of the nucleus of the isotope.
Solution. Let's use the formula
 .
.
Atomic mass of oxygen  =15.9949 amu;
=15.9949 amu;
those. Almost all the weight of an atom is concentrated in the nucleus.
2. Calculate the mass defect and binding energy of the nucleus 3 Li 7 .
Solution.
The mass of the nucleus is always less than the sum of the masses of free (located outside the nucleus) protons and neutrons from which the nucleus was formed. Nuclear mass defect (  m) and is the difference between the sum of the masses of free nucleons (protons and neutrons) and the mass of the nucleus, i.e.
m) and is the difference between the sum of the masses of free nucleons (protons and neutrons) and the mass of the nucleus, i.e.
Where Z– atomic number (number of protons in the nucleus); A is the mass number (the number of nucleons that make up the nucleus); m p , m n , m are the masses of the proton, neutron and nucleus, respectively.
Reference tables always give the masses of neutral atoms, but not nuclei, so it is advisable to transform formula (1) so that it includes the mass M neutral atom.
 ,
,
 .
.
Expressing in equality (1) the mass of the nucleus according to the last formula, we obtain
 ,
,
Noticing that m p +m e =M H, Where M H is the mass of a hydrogen atom, we finally find
Substituting into expression (2) the numerical values of the masses (according to the data of the reference tables), we obtain
By bond energy  The nucleus is called the energy that is released in one form or another during the formation of a nucleus from free nucleons.
The nucleus is called the energy that is released in one form or another during the formation of a nucleus from free nucleons.
In accordance with the law of proportionality of mass and energy
 (3)
(3)
Where With is the speed of light in vacuum.
Proportionality factor With 2 can be expressed in two ways: or

If we calculate the binding energy using off-system units, then

With this in mind, formula (3) takes the form
 (4)
(4)
Substituting the previously found value of the nuclear mass defect into formula (4), we obtain
3.
Two elementary particles, a proton and an antiproton, have a mass  kg each, connecting, turn into two gamma - quantum. How much energy is released during this?
kg each, connecting, turn into two gamma - quantum. How much energy is released during this?
Solution.
We find the energy of a gamma - quantum according to the Einstein formula  , where c is the speed of light in vacuum.
, where c is the speed of light in vacuum.
4. Determine the energy required to separate the 10 Ne 20 nucleus into a 6 C 12 carbon nucleus and two alpha particles, if it is known that the specific binding energies in the 10 Ne 20 nuclei; 6 C 12 and 2 He 4 are respectively: 8.03; 7.68 and 7.07 MeV per nucleon.
Solution. During the formation of the nucleus 10 Ne 20, energy would be released from free nucleons:
W Ne \u003d W c y A \u003d 8.03 20 \u003d 160.6 MeV.
Accordingly, for the nucleus 6 12 С and two nuclei 2 4 He:
W c \u003d 7.68 12 \u003d 92.16 MeV,
W He = 7.07 8 = 56.56 MeV.
Then, during the formation of 10 20 Ne from two 2 4 He nuclei and a 6 12 C nucleus, energy would be released:
 W = W Ne – W c – W He
W = W Ne – W c – W He
W= 160.6 - 92.16 - 56.56 = 11.88 MeV.
The same energy must be spent on the process of splitting the 10 20 Ne nucleus into 6 12 C and 2 2 4 H.
Answer. E = 11.88 MeV.
5 . Find the binding energy of the nucleus of the aluminum atom 13 Al 27, find the specific binding energy.
Solution. The nucleus 13 Al 27 consists of Z=13 protons and
A-Z = 27 - 13 neutrons.
The mass of the nucleus is
m i \u003d m at - Z m e \u003d 27 / 6.02 10 26 -13 9.1 10 -31 \u003d 4.484 10 -26 kg \u003d
27.012 amu
The nuclear mass defect is ∆m = Z m p +(A-Z) m n - m i
Numerical value
∆m = 13 1.00759 + 14 x 1.00899 - 26.99010 = 0.23443 amu
Binding energy Wb = 931.5 ∆m = 931.5 0.23443 = 218.37 MeV
Specific binding energy Wsp = 218.37/27 = 8.08 MeV/nucleon.
Answer: binding energy Wb = 218.37 MeV; specific binding energy W sp \u003d 8.08 MeV / nucleon.
16. Nuclear reactions
Nuclear reactions are the processes of transformation of atomic nuclei, caused by their interaction with each other or with elementary particles.
When writing a nuclear reaction, the sum of the initial particles is written on the left, then an arrow is placed, followed by the sum of the final products. For example,

The same reaction can be written in a shorter symbolic form

When considering nuclear reactions, exact conservation laws:
energy, momentum, angular momentum, electric charge and others. If only neutrons, protons and γ-quanta appear as elementary particles in a nuclear reaction, then the number of nucleons is also preserved in the reaction process. Then the balance of neutrons and the balance of protons in the initial and final states must be observed. For reaction  we get:
we get:
Number of protons 3 + 1 = 0 + 4;
Number of neutrons 4 + 0 = 1 + 3.
Using this rule, one of the participants in the reaction can be identified, knowing the rest. Quite frequent participants in nuclear reactions are α
– particles (  - helium nuclei), deuterons (
- helium nuclei), deuterons (  - nuclei of the heavy isotope of hydrogen, containing, in addition to the proton, one neutron each) and tritons (
- nuclei of the heavy isotope of hydrogen, containing, in addition to the proton, one neutron each) and tritons (  - nuclei of the superheavy isotope of hydrogen, containing two neutrons in addition to the proton).
- nuclei of the superheavy isotope of hydrogen, containing two neutrons in addition to the proton).
The difference between the rest energies of the initial and final particles determines the reaction energy. It can be either greater than zero or less than zero. In a more complete form, the above reaction is written as follows:
Where Q is the reaction energy. To calculate it, using tables of nuclear properties, the difference between the total mass of the initial participants in the reaction and the total mass of the reaction products is compared. The resulting mass difference (usually expressed in amu) is then converted into energy units (1 amu corresponds to 931.5 MeV).
17. Examples of problem solving
1.
Determine the unknown element formed during the bombardment of the nuclei of aluminum isotopes  Al-particles, if it is known that one of the reaction products is a neutron.
Al-particles, if it is known that one of the reaction products is a neutron.
Solution. Let's write the nuclear reaction:
Al+ 
 X + n.
X + n.
According to the law of conservation of mass numbers: 27+4 = A+1. Hence the mass number of the unknown element A = 30. Similarly, according to the law of conservation of charges 13+2 = Z+0 And Z = 15.
From the periodic table we find that this is an isotope of phosphorus  R.
R.
2. What nuclear reaction is written by the equation
 ?
?
Solution.
The numbers next to the symbol of a chemical element mean: below - the number of this chemical element in the table of D.I. Mendeleev (or the charge of this particle), and above - the mass number, i.e. the number of nucleons in the nucleus (protons and neutrons together). According to the periodic table, we notice that the element boron B is in fifth place, helium He is in second, and nitrogen N is in seventh. Particle  - neutron. This means that the reaction can be read as follows: the nucleus of a boron atom with a mass number of 11 (boron-11) after capture
- neutron. This means that the reaction can be read as follows: the nucleus of a boron atom with a mass number of 11 (boron-11) after capture  - particles (one nucleus of a helium atom) emits a neutron and turns into the nucleus of a nitrogen atom with a mass number of 14 (nitrogen-14).
- particles (one nucleus of a helium atom) emits a neutron and turns into the nucleus of a nitrogen atom with a mass number of 14 (nitrogen-14).
3.
When irradiating aluminum nuclei - 27 hard  - magnesium nuclei are formed by quanta - 26. What particle is released in this reaction? Write an equation for a nuclear reaction.
- magnesium nuclei are formed by quanta - 26. What particle is released in this reaction? Write an equation for a nuclear reaction.
Solution.


According to the law of conservation of charge: 13+0=12+Z; 

4. When the nuclei of a certain chemical element are irradiated with protons, sodium nuclei are formed - 22 and - particles (one for each transformation act). What nuclei were irradiated? Write an equation for a nuclear reaction.
Solution.
By periodic system chemical elements of D.I. Mendeleev: 

According to the law of conservation of charge:

According to the law of conservation of mass number:

5 . When the nitrogen isotope 7 N 14 is bombarded with neutrons, the carbon isotope 6 C 14 is obtained, which turns out to be β-radioactive. Write equations for both reactions.
Solution . 7 N 14 + 0 n 1 → 6 C 14 + 1 H 1 ; 6 C 14 → -1 e 0 + 7 N 14.
6. The stable decay product of 40 Zr 97 is 42 Mo 97 . As a result of what radioactive transformations of 40 Zr 97 is it formed?
Solution. Let us write two β-decay reactions that occur sequentially:
1) 40 Zr 97 →β→ 41 X 97 + -1 e 0, X ≡ 41 Nb 97 (niobium),
2) 41 Nb 97 →β→ 42 Y 97 + -1 e 0, Y ≡ 42 Mo 97 (molybdenum).
Answer : as a result of two β-decays, a molybdenum atom is formed from a zirconium atom.
18. Energy of a nuclear reaction
Energy of a nuclear reaction (or thermal effect of a reaction)
Where  is the sum of the masses of the particles before the reaction,
is the sum of the masses of the particles before the reaction,  is the sum of the masses of the particles after the reaction.
is the sum of the masses of the particles after the reaction.
If  , the reaction is called exoenergetic, as it proceeds with the release of energy. At
Q
, the reaction is called exoenergetic, as it proceeds with the release of energy. At
Q
Nuclear fission by neutrons - exoenergetic reaction , in which the nucleus, capturing a neutron, splits into two (occasionally - into three) mostly unequal radioactive fragments, emitting along with this gamma - quanta and 2 - 3 neutrons. These neutrons, if there is enough fissile material around, can in turn cause fission of the surrounding nuclei. In this case, there is chain reaction accompanied by the release a large number energy. Energy is released due to the fact that the fissioning nucleus has either a very small mass defect or even an excess of mass instead of a defect, which is the reason for the instability of such nuclei with respect to fission.
Nuclei, a fission product, have significantly larger mass defects, as a result of which energy is released in the process under consideration.
19. Examples of problem solving
1. What energy corresponds to 1 amu?
Solution . Since m \u003d 1 amu \u003d 1.66 10 -27 kg, then
Q \u003d 1.66 10 -27 (3 10 8) 2 \u003d 14.94 10-11 J ≈ 931 (MeV).
2. Write an equation for a thermonuclear reaction and determine its energy yield, if it is known that the fusion of two deuterium nuclei produces a neutron and an unknown nucleus.
Solution.

according to the law of conservation of electric charge:
1+1=0+Z; Z=2 
according to the law of conservation of mass number:
2+2=1+A; A=3 


 energy is released
energy is released
 =- 0.00352 amu
=- 0.00352 amu
3. During the fission of the uranium nucleus - 235 as a result of capture slow neutron fragments are formed: xenon - 139 and strontium - 94. Three neutrons are simultaneously released. Find the energy released during one act of fission.
Solution. Obviously, during fission, the sum of the atomic masses of the resulting particles is less than the sum of the masses of the initial particles by the value
Assuming that all the energy released during fission is converted into kinetic energy fragments, we get after substituting numerical values:
4. How much energy is released as a result of a thermonuclear fusion reaction of 1 g of helium from deuterium and tritium?
Solution . The thermonuclear reaction of the fusion of helium nuclei from deuterium and tritium proceeds according to the following equation:
 .
.
Define the mass defect
m=(2.0474+3.01700)-(4.00387+1.0089)=0.01887(a.m.u.)
1 amu corresponds to the energy of 931 MeV, therefore, the energy released during the synthesis of the helium atom,
Q=931.0.01887(MeV)
1 g of helium contains  /A atoms, where is the Avogadro number; A is the atomic weight.
/A atoms, where is the Avogadro number; A is the atomic weight.
Total energy Q= (/А)Q; Q=42410 9 J.
5
.
On impact  -particles with a boron nucleus 5 In 10 a nuclear reaction occurred, as a result of which the nucleus of the hydrogen atom and an unknown nucleus were formed. Determine this nucleus and find the energy effect of the nuclear reaction.
-particles with a boron nucleus 5 In 10 a nuclear reaction occurred, as a result of which the nucleus of the hydrogen atom and an unknown nucleus were formed. Determine this nucleus and find the energy effect of the nuclear reaction.
Solution. Let's write the reaction equation:
5 V 10 + 2 Not 4  1 H 1 + z X A
1 H 1 + z X A
From the law of conservation of the number of nucleons it follows that:
10 + 4 + 1 + A; A = 13
From the law of conservation of charge it follows that:
5+2=1+Z; Z = 6
According to the periodic table, we find that the unknown nucleus is the nucleus of the carbon isotope 6 C 13.
The energy effect of the reaction is calculated by the formula (18.1). In this case:
We substitute the masses of isotopes from the table (3.1):
Answer: z X A \u003d 6 C 13; Q = 4.06 MeV.
6. What amount of heat was released during the decay of 0.01 mole of a radioactive isotope in a time equal to half the half-life? During the decay of the nucleus, an energy of 5.5 MeV is released.
Solution. According to the law of radioactive decay:
 =
= .
.
Then, the number of decayed nuclei is equal to:
 .
.
Because  ν 0 , then:
ν 0 , then:
 .
.
Since in one decay an energy equal to E 0 \u003d 5.5 MeV \u003d 8.8 10 -13 J is released, then:
Q = E o N p = N A o E o (1 -  ),
),
Q = 6.0210 23 0.018.810 -13 (1 -  ) = 1.5510 9 J
) = 1.5510 9 J
Answer: Q = 1.55 GJ.
20. Fission reaction of heavy nuclei
Heavy nuclei, when interacting with neutrons, can be divided into two approximately equal parts - fission fragments. Such a reaction is called fission reaction of heavy nuclei , For example
In this reaction, neutron multiplication is observed. The most important quantity is neutron multiplication factor k . It is equal to the ratio of the total number of neutrons in any generation to the total number of neutrons that generated them in the previous generation. Thus, if in the first generation there was N 1 neutrons, then their number in nth generation will
N n = N 1 k n .
At k=1 the fission reaction is stationary, i.e. the number of neutrons in all generations is the same - there is no neutron multiplication. The corresponding state of the reactor is called critical.
At k>1 the formation of a chain uncontrolled avalanche reaction is possible, which occurs in atomic bombs. In nuclear power plants, a controlled reaction is maintained, in which the number of neutrons is maintained at a certain constant level due to graphite absorbers.
Possible nuclear fusion reactions or thermonuclear reactions, when one heavier nucleus is formed from two light nuclei. For example, the synthesis of nuclei of hydrogen isotopes - deuterium and tritium and the formation of a helium nucleus:

At the same time, 17.6 MeV energy, which is about four times more per nucleon than in a nuclear fission reaction. The fusion reaction takes place during the explosions of hydrogen bombs. For more than 40 years, scientists have been working on the implementation of a controlled thermonuclear reaction, which would open access to humanity's inexhaustible “storeroom” of nuclear energy.
21. Biological effect of radioactive radiation
The radiation of radioactive substances has a very strong effect on all living organisms. Even relatively weak radiation, which, when completely absorbed, raises the body temperature by only 0.00 1 ° C, disrupts the vital activity of cells.
A living cell is a complex mechanism that is unable to continue its normal activity even with minor damage to its individual sections. Meanwhile, even weak radiation can cause significant damage to cells and cause dangerous diseases (radiation sickness). At high radiation intensity, living organisms die. The danger of radiation is aggravated by the fact that they do not cause any pain even at lethal doses.
The mechanism of the action of radiation that affects biological objects has not yet been sufficiently studied. But it is clear that it is reduced to the ionization of atoms and molecules, and this leads to a change in their chemical activity. The most sensitive to radiation are the nuclei of cells, especially cells that are rapidly dividing. Therefore, first of all, radiation affects the bone marrow, which disrupts the process of blood formation. Next comes the damage to the cells of the digestive tract and other organs.
atomic Document
Danilova atomiccore Danilov"
Tokens of attention feedback reviews reviews
DocumentThere was no pain in my heart. viola Danilova(in the novel by V. Orlov) was punished with increased ... sees. Yes, it's impossible to understand. atomiccore, not knowing strong interactions, ... on January 2 and 4, I recalled the "violist Danilov", who was punished with the ability to feel everything ...