RADIOACTIVITY, the property of some chemical elements to spontaneous decay into other elements. This decay is accompanied by various corpuscular and light radiations. The phenomenon of radioactivity was discovered in 1896 by G. Becquerel. He discovered that U salts give off radiation that acts on a photographic plate and imparts electrical conductivity to the air. Further research showed that the emission of "Becquerel rays" is an atomic property of U, independent of the chemical compound it is in. Systematic studies of all known elements found that, in addition to U, only Th has significant radioactivity (G. Schmitt and M. Curie, 1898). Subsequently, the still weak radioactivity of K and Rb was discovered. When studying natural compounds of U, it turned out that their radioactivity is much higher than should be expected from the content of U in them. The hypothesis expressed by M. Curie that this anomaly is associated with the presence of unknown highly radioactive elements in uranium minerals was confirmed. Through long and painstaking work, P. and M. Curie managed to isolate new elements Po (1898) and Ra (1898) from the uranium resin ore of the Joachimstal deposit (Czecho-Slovakia), the radioactivity of which is many times greater than the radioactivity of U. This marked the beginning of the discovery of a number of new radioactive elements or radioelements, the number of which reaches 40.
 radioactive radiation. By their nature, the radiation of radioactive elements is not uniform. In 1902, E. Rutherford proposed the names α-(alpha), β-(beta) and γ-(gamma) rays for three types of radioactive radiation with the following properties: α-rays are formed by positively charged fast moving material particles of atomic size and weakly deviate in the electric and magnetic fields in the direction corresponding to the deflection of the channel beams; they are very strongly absorbed by matter; β-rays - particles of negative electricity (electrons); they deviate much more magnetic field and are quite similar to cathode rays. Their permeability is much greater than that of α-rays; γ-rays do not experience deflection in a magnetic field and do not carry a charge; they have the highest permeability (Fig. 1). α-particles carry a double elementary positive charge equal to 9.55·10 -10 CGSE. By their nature, they are identical with the nucleus of the helium atom; α-particles fly out of the atom with a certain initial speed characteristic of each α-radiating radio element; this initial speed lies within 1.4·10 9 -2.06·10 9 cm/s. In air, α-particles, due to their large mass, move almost rectilinearly, gradually wasting their kinetic energy in collisions with gas molecules and causing strong ionization. α-particles have a certain flight range, after which they lose their charge and the ability to cause characteristic actions. The value of this range of flight or "run" of α-particles depends on the initial velocity of the particle and on the absorbing substance. The range of the α-particle is characteristic for each radio element and embraces periods of 2.67-8.62 cm in air at 0°C and 760 mm. IN solidsα-particles are retained by a layer thickness of the order of 0.1 mm. The property of water vapor to condense under certain conditions on ions makes it possible to observe and photograph the paths of α-particles (Fig. 2).
radioactive radiation. By their nature, the radiation of radioactive elements is not uniform. In 1902, E. Rutherford proposed the names α-(alpha), β-(beta) and γ-(gamma) rays for three types of radioactive radiation with the following properties: α-rays are formed by positively charged fast moving material particles of atomic size and weakly deviate in the electric and magnetic fields in the direction corresponding to the deflection of the channel beams; they are very strongly absorbed by matter; β-rays - particles of negative electricity (electrons); they deviate much more magnetic field and are quite similar to cathode rays. Their permeability is much greater than that of α-rays; γ-rays do not experience deflection in a magnetic field and do not carry a charge; they have the highest permeability (Fig. 1). α-particles carry a double elementary positive charge equal to 9.55·10 -10 CGSE. By their nature, they are identical with the nucleus of the helium atom; α-particles fly out of the atom with a certain initial speed characteristic of each α-radiating radio element; this initial speed lies within 1.4·10 9 -2.06·10 9 cm/s. In air, α-particles, due to their large mass, move almost rectilinearly, gradually wasting their kinetic energy in collisions with gas molecules and causing strong ionization. α-particles have a certain flight range, after which they lose their charge and the ability to cause characteristic actions. The value of this range of flight or "run" of α-particles depends on the initial velocity of the particle and on the absorbing substance. The range of the α-particle is characteristic for each radio element and embraces periods of 2.67-8.62 cm in air at 0°C and 760 mm. IN solidsα-particles are retained by a layer thickness of the order of 0.1 mm. The property of water vapor to condense under certain conditions on ions makes it possible to observe and photograph the paths of α-particles (Fig. 2).  β-rays of most radioactive substances form several groups with different initial velocities, the distribution of which is studied by the deflection of β-particles in a magnetic field (β-ray spectrum). The initial speeds of β-particles are in the range of 8.7·10 9 -2.947·10 10 cm/sec, i.e. up to 0.988 of the speed of light. When passing through matter, β-rays scatter much more strongly than α-rays, changing their speed little. Their absorption occurs according to a law close to simple exponential I d \u003d I 0 e - kd, where I d is the intensity of radiation that has passed through the thickness d, I 0 is the initial intensity, k is the absorption coefficient. A characteristic value can be the thickness of a layer of some substance, for example, aluminum, which absorbs β-rays by half. For various β-rays, the size of this layer is 0.001-0.05 cm of aluminum. The hardest RaC β-rays are completely absorbed by two mm of lead, γ-rays are completely similar in nature to X-rays and are characterized by a wavelength of 10 -9 -10 -11 cm. The absorption of γ-rays is accompanied by scattering and the appearance of secondary β- and γ-rays . Approximately, absorption is expressed by a simple exponential law I = I 0 e -μх, and in the first approximation, the relation μ/ϱ = Const is satisfied, where ϱ is the density of the absorbing substance. The value of μ for γ-rays of various radioelements ranges from 1000 to 0.12, which corresponds to the thickness of the Pb layer, which absorbs the rays by half, 10 -4 -5.5 cm.
β-rays of most radioactive substances form several groups with different initial velocities, the distribution of which is studied by the deflection of β-particles in a magnetic field (β-ray spectrum). The initial speeds of β-particles are in the range of 8.7·10 9 -2.947·10 10 cm/sec, i.e. up to 0.988 of the speed of light. When passing through matter, β-rays scatter much more strongly than α-rays, changing their speed little. Their absorption occurs according to a law close to simple exponential I d \u003d I 0 e - kd, where I d is the intensity of radiation that has passed through the thickness d, I 0 is the initial intensity, k is the absorption coefficient. A characteristic value can be the thickness of a layer of some substance, for example, aluminum, which absorbs β-rays by half. For various β-rays, the size of this layer is 0.001-0.05 cm of aluminum. The hardest RaC β-rays are completely absorbed by two mm of lead, γ-rays are completely similar in nature to X-rays and are characterized by a wavelength of 10 -9 -10 -11 cm. The absorption of γ-rays is accompanied by scattering and the appearance of secondary β- and γ-rays . Approximately, absorption is expressed by a simple exponential law I = I 0 e -μх, and in the first approximation, the relation μ/ϱ = Const is satisfied, where ϱ is the density of the absorbing substance. The value of μ for γ-rays of various radioelements ranges from 1000 to 0.12, which corresponds to the thickness of the Pb layer, which absorbs the rays by half, 10 -4 -5.5 cm.
Theory of radioactive decay. In order to explain radioactive phenomena, Rutherford and Soddy proposed in 1902 the theory of atomic decay, which was fully confirmed by further experiments. Atoms of radioactive elements are unstable formations and are subject to spontaneous decay, subject to the law of chance. In this case, intra-atomic energy is released in the form of radiation, while the atom undergoes a transformation, passing into another chemical element with completely different properties, for example, Ra metal turns into RaEm - an inert gas. The basic law of radioactive decay is formulated as follows: the amount of matter ΔN decaying in the element of time Δt is proportional to its present amount N and the time interval Δt, i.e. ΔN=-λNΔt, or N t =N 0 e -Δ t where N 0 - initial quantity, N t - quantity for the moment t. The coefficient of proportionality λ is called the radioactive constant, or the decay constant of a radio element. More clearly, each radioelement is characterized by a half-life, i.e., a period of time during which its initial amount is reduced by half, or by an average life span τ. The half-life T, the average life span τ and the radioactive constant λ are related to each other as follows: T= 0.6931τ = 0.6931 τ -1 . For various radioelements λ = 1.3 10 -13 - 10 11 sec., respectively T=5.2 10 17 sec. (1.65 10 10 years) -10 -11 sec. Between λ and the range of α-rays R there is the relation Ig λ = A + B lg R found empirically by Geiger and Nutall, where A and B are constants. The graphical representation of the law of Geiger and Nutall gives three parallel lines for the three radioactive families U - Ra, Th and Ac. This law has to be used, among other things, to determine the radioactive constants of rapidly decaying substances. The phenomena of radioactive decay, accompanied by the ejection of α- and β-particles from the nucleus of the atom, gave the first evidence of the complex structure of the atomic nucleus, which contains electrons, protons, and He nuclei as structural elements. The patterns observed in the distribution of γ-ray wavelengths and the velocities of β- and α-particles indicate the existence of stable states in the nucleus corresponding to certain energy levels. γ-radiation is apparently associated with intranuclear transitions of α-particles from one energy level to another, and the wavelength of the γ-ray is determined from quantum relations. In a radioactive transformation accompanied by the ejection of an α-particle from the nucleus, it must pass through the level potential energy, which is much greater than the particle's own energy, which it possesses in the nucleus. From point of view classical theory it is impossible to explain the ejection of an α-particle from the nucleus through this "potential barrier". Radioactive decay theories based on the principles of wave mechanics describe the motion of α-particles by means of a wave function, the α-radiation being the result of the gradual penetration of the wave function through the aforementioned potential barrier. In this case, it is possible to find a theoretical expression for the relationship between the velocity of α-particles and the decay constant of the atom, which satisfies the experimental data. Assuming that α-particles in the nucleus of an atom have the same amount of energy with which they leave the nucleus during decay, we obtain the initial value for estimating the absolute values of the energy levels in the nucleus of an atom. These quantities are of the order of 10 6 V (in the notation of atomic physics). β-radiation of radioactive elements form, on the one hand, groups of electrons of certain velocities, most likely appearing as a result of the photoelectric effect caused by γ-radiation of the nucleus in the electron shells of the atom, on the other hand, β-particles emitted from the nucleus have very different velocities values (continuous magnetic spectrum of γ-rays). Often, the α-transformation in the decay series is followed by two β-transformations, which is falsely interpreted as a violation of the stability of the electronic levels in the nucleus with a decrease in the number of α-particles. The energy relationships in the nucleus associated with β-radiation do not yet appear to be quite clear.
When a radioactive atom decays, b. h. also a radioactive element. That. decay series are formed, or radioactive families, successively transforming radioelements. The law of radioactive decay makes it possible to calculate the number of any of the members of the series for each moment of time under given initial conditions. In practice, the most important cases are the following. 1) Decay of a separate radio element, for example RaEm; the amount of a radio element at any moment will be expressed as follows: N t =N 0 e -λ t initial amount (at t=0). 2) Formation from a radio element with a very long lifespan (the amount of which practically does not change over the considered period of time, for example, the formation of UX (half-life 24 days) from U (half-life 10 9 years). In this case, the number of atoms of the resulting element N 2 for the moment t is expressed in terms of the number of atoms of the parent element N 1 and the corresponding decay constants as follows:
3) The case of radioactive equilibrium, when a constant ratio of the numbers of atoms of successive elements in the decay series is preserved. In this case, the following equalities are observed: N 1 λ=N 2 λ 2 =…=N k λ k if the series in question contains k elements (Fig. 3, A - growth and B - decay of ThX). The transformation of radio elements is always accompanied by α- or β-radiation. Not a single case is known when a radioactive transformation would be accompanied by only one γ-radiation.

The study of radioactive transformations led to the discovery of a large number of new elements. When trying to place radioelements in the periodic table, difficulties arose, since the number of free places turned out to be insufficient. These difficulties were overcome as a result of studying the chemical characteristics of radioelements. Boltwood, who discovered the new radioactive element ionium in 1906, showed that his Chemical properties completely coincide with the properties of the element thorium. Further, similar chemical identity was found in a number of radioelements (Ra and MsTh, Pb, RaB, ThB, AsB, etc.), and in 1910 Soddy suggested that these elements have fundamentally the same properties, and their separation by chemistry is impossible. A group of such chemically indistinguishable elements is called, according to the proposal of Faience, a pleiad, and the elements themselves, according to the proposal of Soddy, isotopes, because. they occupy the same place in the periodic table. At the same time, Soddy suggested that non-radioactive elements could also be a mixture of fundamentally inseparable elements of different atomic weights, which explains the fractional values of the atomic weights of most elements. This idea of Soddy found a brilliant confirmation in the works of Aston, who discovered isotopes of ordinary elements by the method of positive rays. The concept of isotopy made it possible to place all radioelements in the periodic system. They embrace 10 pleiades located in the last two rows periodic system(Fig. 4).

The characteristic elements, or dominants, of the galaxy of radioactive isotopes are the elements with the longest lifespan, or stable elements. At the same time, five of them: Ra, Em, Po, Ac and Ra are new elements that have taken free places in the periodic system, while the rest fall into places occupied by the previously known radio elements U and Th and inactive Pb, Tl, Bi. The largest difference in the atomic weights of radioactive isotopes does not exceed 8 units. That. radioactive transformations made it possible to delve deeper into physical meaning periodic law and the concept of a chemical element. It turned out that the place of an element in the periodic system is determined not by the atomic weight of the element, as was previously accepted, but by the value of the positive charge of the nucleus of its atom. All properties of isotopes associated with the electron shells of an atom are practically the same within the accuracy of our experiments (atomic volume, transition temperature from one state to another, thermal change in size, magnetic susceptibility, spectra, etc.). Apart from their radioactive properties, they differ only in those features that are associated with the mass of the nucleus, for example, in the fine structure of the spectrum and in negligible differences in the diffusion constants. The last circumstance is the basis for attempts to separate isotopes, which, as a result of painstaking work, led to partial success.
During radioactive decay, the transformation of elements occurs, subject to the following shift rules (K. Fajans). 1) After the emission of an α-particle, the element is shifted two places to the left in the periodic table. 2) During the β-transformation, the element is shifted one place to the right (the direction of the arrows in Fig. 4). These rules indicate that radioactivity is a property of the nucleus of an atom, since the emission of an α-particle carrying two elementary positive charges reduces the charge of the nucleus by two units, which corresponds to a decrease in the atomic number by two units. The β-particle carries away one negative charge, i.e., increases the positive charge of the nucleus, and, consequently, its atomic number by one. As a result of radioactive transformations, two different elements can occupy the same place in the periodic table.
All radioelements known to us form three radioactive families, or series: the U-Ra family, the Ac family, and the Th family. The U and Th series are independent, while the Ac series, according to all data, is connected with the U-Ra series. In FIG. 5 shows a diagram of radioactive families with their transformations. Radioelements Ra and MsTh are of the greatest practical interest, as they have a very high radioactivity and are a source of highly radioactive elements with a short lifespan (for example, RaEm, ThX, etc.). Of the other chemical elements, only K and Rb have weak radioactivity with the emission of β- and γ-rays. Their transformation products are unknown.

Actions of radioactive radiation. 1) All radioactive emissions produce ionization of gases. In this case, α-rays act most strongly, the action of β- and γ-rays is much weaker. To a lesser extent, ionization is observed in liquid and solid dielectrics. 2) The energy of radioactive radiation is converted into heat when their matter is absorbed. In this case, the greatest effect is also given by α-rays, which have the maximum energy. Theoretically, the amount of heat released can be calculated, knowing the energy of radiation and the kinetic energy of the rest of the decayed atom. Experimentally, the thermal effect has been especially carefully studied for Ra; 1 g of Ra releases 25 cal per hour, and together with the decay products 170 cal. 3) Strong radioactive preparations glow themselves and cause a number of bodies to glow. Flashes on the screen of zinc sulfide, caused by individual α-particles ( scintillations), make it possible to count α-particles emitted by radio elements. 4) Many substances change their color under the influence of radioactive radiation. 5) Radioactive rays act on a photographic plate. By applying a poorly polished surface of a piece of radioactive ore to a photographic plate, one can obtain radiography of the distribution of radioactive minerals over the surface of the sample. 6) Under the action of radioactive radiation, chemical reactions, associated mainly with the ionization they cause; some of the effects of β-rays on colloids are explained by the negative charge of the β-particles themselves. 7) The action of radioelements on a living organism is manifested in the form of local and general phenomena and strongly depends on the dose. The action of radioactive radiation is expressed in the general fatigue of the body, changes in the composition of the blood (decrease in the number of white blood cells, etc.). With local exposure to β-rays of large amounts of radioelements, a burn can occur, which is difficult to heal. Young cells are most sensitive to the action of radiation. The introduction of large amounts of radioelements into the body leads to death. Small amounts of radioelements have a beneficial effect on the body.
Practical applications of radioactivity. 1) The property of radio elements to ionize gases has found its application in the manufacture of radioactive collectors used to measure electric field, mainly in the study of atmospheric electricity. For this purpose, α-emitters Io or Bo are usually used. The latter has to be periodically renewed, because it breaks up by half in 137 days. 2) Radioactive Em m. b. used in determining the gas permeability of various substances. 3) When α-particles pass through various substances, under certain conditions, the appearance of H-particles (hydrogen nuclei) is observed. This phenomenon was first observed in 1919 by Rutherford in nitrogen and interpreted as the result of the destruction of the nitrogen nucleus in a collision with an α-particle. Further work, mainly by Rutherford's collaborators - Kirsch and Peterson - showed that a large number of elements are destroyed under the action of α-particles. About others practical applications see radioactivity. Radium.
For the quantitative measurement of radioactive substances, the method based on ionization is used almost exclusively. In the case of very strong preparations, it is possible to use a sensitive galvanometer to measure the ionization currents. To measure small amounts of radioelements, electroscopes and electrometers are used. The most important schemes of the devices used are shown in Fig. 6.

1) α-beam measurements. The investigated substance of radioactivity is placed in a finely divided form in a flat cup at the bottom of the "ionization chamber" of the electroscope (Fig. 6a) or electrometer (Fig. 6b). The ionization current is measured by the rate of fall of the electroscope leaf, counted on the ocular scale of the microscope. In this case, it is necessary to take into account the proper fall of the sheet under the influence of defects in the insulation and ionization of the air inside the device, which is determined by special observation in the absence of a radioactive substance. When measuring with an electrometer, either the charging method or compensation methods are used. When measuring along α-rays, a layer of a substance with a thickness of the order of 1 mm is usually taken. Such a layer will be saturated for α-radiation, i.e. α-rays from the lower parts are already absorbed in the very active substance and don't come out. In this case, the measured ionization is approximately proportional to the concentration of radioelements in the preparation. Typically, measurements are made in comparison with a standard containing a known amount of the radioelement being determined, for example, U in equilibrium with decay products. Or the results are expressed in uranium units, and the uranium unit means one-sided radiation of 1 cm 2 of a layer of uranium oxide U 3 O 8 saturated for α-rays. In absolute terms, this corresponds to a saturation current of 1.73·10 -3 CGSE. In the case of an infinitely thin layer (for example, active plaque obtained in the presence of emanations on solids and consisting of their decay products), ionization is proportional to the amount of radio element on the preparation. 2) γ-ray measurements. Due to the high penetrability of γ-rays, it is possible to measure the amount of radio elements (usually Ra, RaEm or MsTh) in hermetically sealed preparations with their help. Measurements are made against a standard containing a known amount of Ra. When measuring small amounts of Ra of the order of 10 -5 -10 -7 g, they are placed inside the device of a special device. When measuring large quantities - from 10 -4 g and more - the test preparation is placed at some distance outside the device. 3) Measurements of small amounts of RaEm are made by α-rays in an electrometer with an ionization chamber, adapted for the introduction of Em inside it. Usually you have to measure Em from aqueous solution, while Em is distilled into the ionization chamber with air flow by circulation (Fig. 7) or in some other way.

Next, the ionization caused by α-rays of Em and products of its water sources is measured. The same method is used to determine small amounts of Ra in solution. The test solution is placed in a gas-washing flask L and freed from Em by blowing air through it for 10–30 min. Then the vessel with the solution is hermetically sealed and left for several days for the accumulation of Em. Next, Em is transferred to the measuring device J, where its amount is determined. The accumulation of emanation occurs according to the formula E=E ∞ (1 e λ t), where E is the amount of Em accumulated during time t, E ∞ is the amount of it that is in equilibrium with radium in a given solution. Numerically, E ∞ is equal to as many curies of emanation as grams of Ra are in solution.
The standard is a solution with a known Ra content of the order of 10 -8 -10 -9 g. From Em it is possible to measure 10 -10 g even 10 -12 g of Ra. 4) Measurement of the number of individual particles is carried out either using the scintillation method or by appropriately amplifying the ionizing effect of individual particles or pulses (Geiger counter). It is also possible to use a photographic plate with a thick layer (method of L. V. Mysovsky).
- rays of the first type are deflected in the same way as a stream of positively charged particles; they were called α-rays;
- rays of the second type deviate in a magnetic field in the same way as a stream of negatively charged particles (in the opposite direction), they were called β-rays;
- rays of the third type, which are not deflected by a magnetic field, are called γ-radiation.
Alpha decay
α-decay called the spontaneous decay of the atomic nucleus into a daughter nucleus and an α-particle (the nucleus of the 4 He atom).
α-decay, as a rule, occurs in heavy nuclei with a mass number A≥140 (although there are a few exceptions). Inside heavy nuclei, due to the property of saturation of nuclear forces, separate α-particles are formed, consisting of two protons and two neutrons. The resulting α-particle is subject to a greater action of the Coulomb repulsive forces from the protons of the nucleus than individual protons. At the same time, the α-particle experiences less nuclear attraction to the nucleons of the nucleus than the rest of the nucleons. The resulting alpha particle at the boundary of the nucleus is reflected inward from the potential barrier, but with some probability it can overcome it (see tunnel effect) and fly out. As the energy of the alpha particle decreases, the permeability of the potential barrier decreases exponentially, so the lifetime of nuclei with a lower available energy of alpha decay, other things being equal, is longer.
Soddy's shift rule for α-decay:
. .As a result of α-decay, the element is shifted 2 cells to the beginning of the periodic table, the mass number of the daughter nucleus decreases by 4.
beta decay
Becquerel proved that β-rays are a stream of electrons. β decay is a manifestation of the weak force.
β-decay(more precisely, beta minus decay, β - decay) is a radioactive decay, accompanied by the emission of an electron and an antineutrino from the nucleus.
β decay is an intranucleon process. It occurs as a result of the transformation of one of d-quarks in one of the neutrons of the nucleus in u-quark; in this case, the neutron is converted into a proton with the emission of an electron and an antineutrino:
Soddy's shift rule for β − decay:
After β − -decay, the element is shifted by 1 cell to the end of the periodic table (the nuclear charge increases by one), while the mass number of the nucleus does not change.
There are also other types of beta decay. In positron decay (beta plus decay), the nucleus emits a positron and a neutrino. In this case, the charge of the nucleus decreases by one (the nucleus is shifted one cell to the beginning of the periodic table). Positron decay Always accompanied by a competing process - electron capture (when the nucleus captures an electron from the atomic shell and emits a neutrino, while the charge of the nucleus also decreases by one). However, the converse is not true: many nuclides, for which positron decay is forbidden, experience electron capture. The rarest known type of radioactive decay is double beta decay, it has been found to date for only ten nuclides, and half-lives exceed 10 19 years. All types of beta decay conserve the mass number of the nucleus.
Gamma decay (isomer transition)
Almost all nuclei have, in addition to the ground quantum state, a discrete set of excited states with higher energy (exceptions are the nuclei ¹H , ²H , ³H and ³He). Excited states can be populated during nuclear reactions or radioactive decay of other nuclei. Most excited states have very short lifetimes (less than a nanosecond). However, there are also sufficiently long-lived states (whose lifetimes are measured in microseconds, days, or years), which are called isomeric states, although the boundary between them and short-lived states is very arbitrary. The isomeric states of nuclei, as a rule, decay into the ground state (sometimes through several intermediate states). In this case, one or more gamma quanta are emitted; the excitation of the nucleus can also be removed by the emission of conversion electrons from the atomic shell. Isomeric states can also decay through the usual beta and alpha decays.
Special types of radioactivity
- Proton radioactivity
- Two-proton radioactivity
- Neutron radioactivity
Literature
- Sivukhin D.V. General course of physics. - 3rd edition, stereotypical. - M .: Fizmatlit, 2002. - T. V. Atomic and nuclear physics. - 784 p. - ISBN 5-9221-0230-3
see also
- Radioactivity units
Wikimedia Foundation. 2010 .
Synonyms:See what "Radioactivity" is in other dictionaries:
Radioactivity ... Spelling Dictionary
- (from Latin radio I radiate, radius beam and activus effective), the ability of some at. nuclei spontaneously (spontaneously) turn into other nuclei with the emission of h c. Radioactive transformations include: alpha decay, all types of beta decay (with ... ... Physical Encyclopedia
RADIOACTIVITY- RADIOACTIVITY, a property of some chem. elements spontaneously transform into other elements. This transformation or radioactive decay is accompanied by the release of energy in the form of various corpuscular and radiant radiation. The phenomenon of R. was ... ... Big Medical Encyclopedia
Radioactivity- (from radio ... and Latin activus active), property atomic nuclei spontaneously (spontaneously) change its composition (nuclear charge Z, number of nucleons A) by emitting elementary particles, g quanta or nuclear fragments. Some of… … Illustrated Encyclopedic Dictionary
- (from Latin radio I emit rays and activus effective) spontaneous transformation of unstable atomic nuclei into nuclei of other elements, accompanied by the emission of particles or? quantum. 4 types of radioactivity are known: alpha decay, beta decay, ... ... Big Encyclopedic Dictionary
The ability of some atomic nuclei to spontaneously decay with the emission of elementary particles and the formation of the nucleus of another element. R. uranium was first discovered by Becquerel in 1896. Somewhat later, M. and P. Curie and Rutherford proved ... ... Geological Encyclopedia
Some property. bodies to emit a special kind of invisible rays, differing special properties. Dictionary of foreign words included in the Russian language. Chudinov A.N., 1910. radioactivity (radio ... + lat. activus active) radioactive ... ... Dictionary of foreign words of the Russian language
Exist., number of synonyms: 1 gamma radioactivity (1) ASIS Synonym Dictionary. V.N. Trishin. 2013 ... Synonym dictionary
Spontaneous transformation of unstable isotopes of one chemical element into isotopes, usually of another element, accompanied by the emission of elementary particles or nuclei (alpha and beta radiation), as well as gamma radiation. It happens natural and ... ... Marine dictionary
The main types of radioactivity are alpha, beta and gamma decays.
Alpha decay. In this case, spontaneous emission of an α-particle (nucleus of the nuclide 4He) occurs by the nucleus, and this occurs according to the scheme
where X is the symbol of the parent nucleus, Y is the symbol of the child.
It has been established that α-particles emit only heavy nuclei. The kinetic energy with which α-particles fly out of the decaying nucleus is on the order of several MeV. In air at normal pressure, the range of α-particles is several centimeters (their energy is spent on the formation of ions on their way).
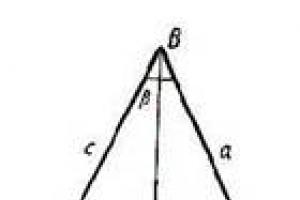
An alpha particle appears only at the moment of radioactive decay of the nucleus. Leaving the core, it has to overcome the potential barrier
ep, the height of which exceeds its energy (see figure).
The inner side of the barrier is due to nuclear forces, while the outer side is due to the forces of the Coulomb repulsion of the α-particle and the daughter nucleus.
Overcoming potential by α-particle
barrier under these conditions is due to the tunnel effect
Quantum theory, taking into account the wave properties of the α-particle, "allows" it to penetrate such a barrier with a certain probability. The corresponding calculation is well confirmed by the measurement results.
beta decay . This is the name of a spontaneous process in which the original nucleus turns into another nucleus with the same mass number A, but with charge number Z, which differs from the original by ±1. This is due to the fact that β -decay is accompanied by the emission of an electron (positron) or its capture from the shell of an atom. There are three varieties β -decay:
1)electronic- decay in which the nucleus emits an electron and its charge number Z becomes Z + 1;
2) positron - decay in which the nucleus emits a positron and its charge number Z becomes Z - 1;
3)TO-capture, in which the nucleus captures one of the electrons in the electron shell of the atom (usually from TO-shells) and its charge number Z becomes equal Z-1. to the vacant seat in TO-shell-ke passes an electron from another shell, and therefore TO-seizure is always accompanied by character-
static x-rays.
The “-decay problem” was solved by Pauli (1930), who suggested that an electrically neutral particle is emitted together with an electron, which is elusive due to its very large penetrating power. They called it the neutrino.
An important circumstance in favor of the hypothesis of the existence of neutrinos is the need to preserve the angular momentum in the decay reaction. The fact is that the distinguishing feature of (-decay is the transformation of a neutron into a proton in the nucleus, and vice versa. Therefore, we can say that -decay is not an intranuclear process, but an intranucleon process. In this regard, the above three types of -decay are due to the following transformations nucleons in the nucleus:
It has now been established that the neutrino spin is 1/2.
Observing neutrinos directly is very difficult. This is because their electrical charge zero, the mass (if any) is extremely small, fantastically small and the effective cross section of their interaction with the nuclei. According to theoretical estimates, the mean free path of a neutrino with an energy of 1 MeV in water is about 10 16 km (or 100 light years!). This is much larger than the stars. Such neutrinos freely penetrate the Sun, and even more so the Earth.
To register the process of neutrino capture, it is necessary to have huge neutrino flux densities. This became possible only after the creation nuclear reactors, which were used as powerful sources of neutrinos.
Direct experimental proof of the existence of the neutrino was obtained in 1956.
Gamma decay. This type of decay consists in the emission of γ-quanta by an excited nucleus during its transition to the normal state, the energy of which varies from 10 keV to 5 MeV. It is essential that the spectrum of emitted γ-quanta is discrete, since the energy levels of the nuclei themselves are discrete.
Unlike β -decay, γ -decay - the process is intranuclear, not intranucleon.
Excited nuclei are formed when β -decay if the decay of the parent nucleus X to the ground state of the child kernel Y prohibited. Then the child nucleus Y turns out to be in one of the excited states, the transition from which to the ground state is accompanied by the emission of y-quanta (see Fig.).
An excited nucleus can also pass to the ground state in another way, by directly transferring the excitation energy to one of the atomic electrons, for example, in TO-shell. This process competes with β -decay is called internal electron conversion. Internal conversion is accompanied by X-rays.
Nuclear reactions
A nuclear reaction is a process of strong interaction of an atomic nucleus with an elementary particle or with another nucleus, a process accompanied by the transformation of nuclei. This interaction arises due to the action of nuclear forces when particles approach each other up to distances of the order of 10 -13 cm.
Note that it is nuclear reactions that provide the most extensive information about the properties of nuclei. Therefore, the study of nuclear reactions is the most important task of nuclear physics.
The most common type of nuclear reaction is the particle interaction A with core x, resulting in the formation of a particle b and core Y. This is written symbolically like this:
Role of Particles A And b most often perform a neutron P, proton R, deuteron d, α -particle and γ -quantum..
Particles produced as a result of a nuclear reaction can be not only b And Y, but along with them other b", Y". In this case, the nuclear reaction is said to have several channels, with different channels corresponding to different probabilities.
Types of nuclear reactions. It has been established that reactions caused by not very fast particles proceed in two stages. The first stage is the capture of the incident particle A core X with the formation of a compound (or intermediate) nucleus. In this case, the energy of the particle A is quickly redistributed among all the nucleons of the nucleus, and the compound nucleus is in an excited state. The nucleus stays in this state until, as a result of internal fluctuations, one of the particles (which may consist of several nucleons) concentrates energy sufficient for its escape from the nucleus.
Such a mechanism for the occurrence of a nuclear reaction was proposed by N. Bohr (1936) and subsequently confirmed experimentally. These reactions are sometimes written with a compound nucleus WITH, such as
where is the star WITH indicates that the kernel WITH* occurs in an excited state.
Compound core WITH* exists for a long time - in comparison with the "nuclear time", i.e., the time of flight of a nucleon with an energy of the order of 1 MeV ( v 10 9 cm/s) of a distance equal to the diameter of the nucleus. nuclear time i 10 -21 s. The lifetime of a compound nucleus in an excited state is ~ 10 -14 s. That is, on a nuclear scale, the compound nucleus lives indeed for a very long time. During this time, all traces of the history of its formation disappear. Therefore, the decay of the compound nucleus - the second stage of the reaction - proceeds regardless of the method of formation of the compound nucleus.
Reactions caused by fast particles with energies exceeding tens of MeV proceed without the formation of a compound nucleus. And the nuclear reaction is usually direct. In this case, the incident particle directly transfers its energy to some particle inside the nucleus, for example, one nucleon, deuteron, α -particle, etc., as a result of which this particle flies out of the nucleus.
A typical direct interaction reaction is a stall reaction, when the incident particle is, for example, a deuteron. When one of the deuteron nucleons enters the area of action of nuclear forces, it will be captured by the nucleus, while the other nucleon of the deuteron will be outside the area of action of nuclear forces and will fly past the nucleus. Symbolically, the breakdown reaction is written as ( d,n) or ( d, p).
When nuclei are bombarded by strongly interacting particles with very high energy (from several hundred MeV and above), the nuclei can "explode", disintegrating into many small fragments. When registered, such explosions leave a trail in the form of multi-beam stars.
Reaction energy. It is customary to say that nuclear reactions can occur both with the release and absorption of energy.
Reactions with the release of energy are called exoenergetic, reactions with the absorption of energy are called endoenergetic.
The electron has antiparticle - positron, which was found in cosmic radiation. The existence of positrons has also been proven by observing their tracks in a cloud chamber placed in a magnetic field. Positron- a particle with a mass equal to the mass of an electron and spin 1/2 (in units) that carries a positive charge + e.
According to Bohr, nuclear reactions proceed in two stages according to the scheme:
The first stage is the capture of a particle by the nucleus A and the formation of an intermediate nucleus WITH, called the composite, or compound-kernel. The second stage is the decay of the compound nucleus into a nucleus Y and particle b.
Frédéric and Irene Joliot-Curie bombarded α -particles B, A1 and Mg, which led to artificially radioactive nuclei undergoing -decay (positron decay or + p- decay):
In nuclear reactions, the displacement rule is satisfied
Process p+- the decay proceeds as if one of the protons of the nucleus turned into a neutron, while emitting a positron and a neutrino:
Positrons can be created in the interaction γ -quanta great energy (E γ> 1.02 MeV = 2 m e s 2) with matter. This process proceeds according to the scheme
Electron-positron pairs were found in a cloud chamber placed in a magnetic field, in which they deviated in opposite directions. The process of transformation of an electron-positron pair (in a collision of a positron with an electron) into two γ - quantum, is called annihilation. During annihilation, the energy of the pair is converted into the energy of photons
The appearance in this process of two γ -quanta follows from the laws of conservation of momentum and energy.
The capture by the nucleus of an electron from one of the inner shells of the atom (K, L, etc.) with the emission of a neutrino (electron capture or e-capture) occurs according to the following scheme:
(the appearance of neutrinos follows from the law of conservation of spin). In general, the scheme e-capture:
Depending on the speed (energy), neutrons are divided into slow and fast.
slow neutrons: ultracold (≤ 10 -7 eV),
very cold (10 -7 ÷10 -4 eV), cold (10 -4 ÷10 -3 eV),
thermal (10 -3 ÷ 0.5 eV), resonant (0.5 ÷ 10 4 eV) Electronic capture is detected by the accompanying characteristic X-rays that arises when the formed vacancies in the electron shell of the atom are filled. All the decay energy is carried away by the neutrino.
Neutrons can be slowed down by passing them through a substance containing hydrogen (for example, water). They experience scattering and slow down.
Radioactivity is the spontaneous decay of unstable atomic nuclei. It is accompanied by the emission of elementary particles or helium nuclei (α-particles) and the transformation of an isotope of one element into an isotope of another.
Radioactive families of thorium-232, uranium-235 and uranium-238.
The French scientist Antoine Becquerel in the summer of 1835 in Venice observed the exceptionally beautiful phosphorescence of the Adriatic Sea. After 61 years, this phenomenon served as one of the guiding threads that allowed his grandson Henri Becquerel to discover the phenomenon of radioactivity. The rays discovered by W. Roentgen in 1895 also attracted the attention of Henri Becquerel because they caused the phosphorescence of various substances. It has been suggested that phosphorescence, in turn, is accompanied by the emission of x-rays. Wanting to test this assumption, Henri Becquerel investigated the double sulfate of uranyl and potassium, a highly phosphorescent compound. It turned out that even without preliminary illumination it emits rays of a previously unknown nature.
Henri Becquerel made this observation on March 1, 1896. In May, he found out that the element uranium, at that time the last element in the periodic table of chemical elements, was responsible for the emission of new rays.
M. Sklodowska-Curie called these rays radioactive, and the very phenomenon of their emission - radioactivity. She also discovered this phenomenon in thorium and, together with her husband P. Curie, isolated two new radioactive elements from uranium minerals - polonium and radium. Beginning in 1899, various scientists began to discover new radioactive substances all over the world. large quantities, for example actinium, emanations (see Radon), etc. As a rule, these substances had very short half-lives (the time during which half of any radioactive substance decays), and therefore scientists even doubted whether these substances were chemical elements in the usual sense . Moreover, the number of free places in the periodic system between bismuth and uranium was very limited.
A huge contribution to the study of radioactivity was made by the English scientist E. Rutherford. Together with the English radiochemist F. Soddy, he proved that radioactivity is accompanied by spontaneous conversion of chemical elements. For example, radium, emitting an a-particle, turns into radon. By 1913, the abundance of radioactive substances (about 40) was reduced to three radioactive families, which are chains of successive convertibility of the ancestors of the series (uranium-238, uranium-235 and thorium-232) into stable lead (see Radioactive elements). Among the radioactive substances were several groups of substances, chemically indistinguishable, but different in mass. They were called isotopes. The discovery of radioactive elements was actually the discovery of individual natural radioactive isotopes: after all, all members of the radioactive families are isotopes of uranium, thorium, protactinium, actinium, radium, radon, polonium, lead. At the same time, all stable elements were originally discovered as natural mixtures of isotopes.
There are several types of radioactive transformations. These are α‑decay (emission of an α‑particle), β −‑decay (emission of an electron) and spontaneous nuclear fission. The emission of γ-rays is not a type of radioactive decay (in this case, the transformation of elements does not occur), but is electromagnetic radiation small wavelengths. These species are observed in nature.
In 1934, the spouses I. and F. Joliot-Curie discovered the phenomenon artificial radioactivity. As a result of nuclear reactions, artificial radioactive isotopes of all elements of the periodic system can be obtained. They are now known about 1800. The study of artificial radioisotopes made it possible to discover new types of radioactive convertibility: positron emission, or β + -decay, and K-capture (absorption of an electron from the nearest electron K-shell by the nucleus) (see Atom). The possibility of proton (emission of a proton) and two-proton (emission of two protons simultaneously) radioactivity has been predicted and proved.
In 1982, American scientists experimentally proved that some nuclei are capable of emitting two protons at once. This is the so-called two-proton radioactivity, which was predicted back in 1960 by the Soviet physicist V.I. Goldansky. And at the end of 1983 English physicists G. Rose and G. Jones discovered a completely amazing type of radioactivity - the emission of heavy particles - 14 C nuclei - by nuclei of the 223 Ra isotope. This discovery aroused great interest and gave rise to an extensive cycle of research in different countries, including the USSR. It turned out that in addition to "carbon" radioactivity, there is also "neon" radioactivity: the nuclei of some isotopes of protactinium and uranium, in addition to their usual α-activity, are capable of emitting neon nuclei. The new kind radioactivity is called "fragmentary" or cluster. Currently, only eight nuclei are known to emit carbon or neon nuclei. These are four isotopes of radium (carbon nuclei fly out) and four isotopes of uranium and protactinium (neon nuclei). Experiments in this area are rapidly developing. Theorists still do not have a single point of view in explaining this rare but extremely interesting type of radioactive decay. Probably, in the arsenal of nature there is still more ways of radioactive decays than we currently imagine.
The phenomenon of radioactivity is characterized by three factors: 1) the rate of radioactive decay; 2) the type of emitted particles and 3) their energy. The decay rate is expressed by a simple mathematical formula:
N t = N 0 e −λt .
In it, N t is the number of atoms of a radioactive element at time t; N 0 is the number of atoms at the initial time (t = 0), e is the base of natural logarithms, and λ is the so-called radioactive decay constant. It is related to the half-life T by:
The half-lives of known radioactive isotopes lie in a very wide time interval - from thousandths of a second to billions of years. However, most isotopes have half-lives ranging from 30 s to 10 days.
The most common type of radioactive transformation is the emission of electrons, or β - decay. It is characteristic of 45% of all known radioactive isotopes and is observed in nuclei with an excess of neutrons, i.e., in heavy radioactive isotopes of elements. More than 15% of radioactive nuclei decay by emitting α-particles; α-decay undergoes isotopes of the elements of the end of the periodic system (starting with bismuth), as well as some elements of its middle (starting with rare earths). For lighter elements, alpha decay is energetically impossible.
Spontaneous fission occurs naturally in the 238 U and 232 Th isotopes; it becomes significant for isotopes of transuranium elements as Z - the charge of the atomic nucleus increases.
Positron decay and K-capture are actually observed only in artificial radioactive isotopes and are characteristic of nuclei with a lack of neutrons. About 10% of isotopes are subject to β + -decay (mostly isotopes of the elements of the first half of the periodic system). The share of electron capture accounts for approximately 25% of the observed radioactive convertibility (they are more characteristic of the isotopes of the elements of the second half of the periodic table, in the atoms of which the inner electron shells are located close to the nucleus).
The study of radioactivity has played a huge role in creating modern ideas about the structure and properties of matter.
The instability of atoms was discovered at the end of the 19th century. After 46 years, the first nuclear reactor was built.
radioactivity called the ability of unstable nuclei to transform into other nuclei, while the transformation process is accompanied by the emission of various particles.
The discovery of radioactivity, a phenomenon that proves the complex composition of the nucleus, happened due to a happy accident. X-rays were first obtained in the collision of fast electrons with the glass wall of the discharge tube. Simultaneously, the glow of the tube walls was observed. becquerel He wrapped the photographic plate in thick black paper, put in the salts, and exposed it to a bright light. After development, the plate turned black in those areas where the salt lay. Consequently, uranium created some kind of radiation, which, like X-rays, penetrates opaque bodies and acts on the plate. Becquerel thought that radiation occurs under the influence of sunlight. But one day, in February 1884, it was not possible to conduct another experiment due to cloudy weather. Becquerel put the record back in a drawer, placing on top of it a copper cross covered with uranium salt. Having developed the plate, just in case, two days later, he found blackening on it in the form of a distinct shadow of a cross. This meant that uranium salts spontaneously, without any external influences, create some kind of radiation.
In 1898 Maria Sklodowska-Curie in France, other scientists also discovered the radiation of thorium. Subsequently, the main efforts in the search for new elements were made Marie Skłodowska-Curie and her husband Pierre Curie. Another element was discovered that gives very intense radiation. It was named radium. The very phenomenon of spontaneous radiation was called by the Curies spouses radioactivity.
Subsequently, it was found that all chemical elements with a serial number greater than 83 are radioactive.
After the discovery of the radioactivity of the elements, the study of the physical nature of their radiation began. In addition to Becquerel and the Curies, Rutherford did this.
The classical experiment that made it possible to detect the complex composition of radioactive radiation was as follows. The radium preparation was placed at the bottom of a narrow channel in a piece of lead. A photographic plate was placed against the canal. The radiation leaving the channel was affected by a strong magnetic field, the lines of induction of which were perpendicular to the beam. The entire setup was placed in a vacuum.
In the absence of a magnetic field, a single dark spot was found on the photographic plate after development, exactly opposite the channel. In a magnetic field, the beam split into three beams. The two components of the primary flow were deviated in opposite sides. This indicated the presence of these radiations electric charges opposite signs. In this case, the negative component of the radiation was deflected by the magnetic field much more than the positive one. The third component was not deflected by the magnetic field. The positively charged component is called alpha rays, the negatively charged component is called beta rays, and the neutral component is called gamma rays.
These three types of radiation are very different from each other in penetrating power, i.e. according to how intensively they are absorbed by various substances.
alpha radiation is a stream of heavy positively charged particles. Arises as a result of the decay of atoms of heavy elements such as uranium, radium and thorium. In the air, alpha radiation travels no more than five centimeters and, as a rule, is completely blocked by a sheet of paper or the outer dead layer of the skin. However, if a substance that emits alpha particles enters the body with food or air, it irradiates the internal organs and becomes dangerous.
beta radiation- these are electrons, which are much smaller than alpha particles and can penetrate several centimeters deep into the body. You can protect yourself from it with a thin sheet of metal, window glass and even ordinary clothing. Getting to unprotected areas of the body, beta radiation has an effect, as a rule, on the upper layers of the skin. During the accident on Chernobyl nuclear power plant in 1986, firefighters suffered skin burns from very high exposure to beta particles. If a substance that emits beta particles enters the body, it will irradiate the internal tissues.
Gamma radiation are photons, i.e. electromagnetic wave that carries energy. In the air, it can travel long distances, gradually losing energy as a result of collisions with the atoms of the medium. Intense gamma radiation, if not protected from it, can damage not only the skin, but also internal tissues. Dense and heavy materials such as iron and lead are excellent barriers to gamma radiation.
Question